Ionis shares fell 7.7% in morning trading.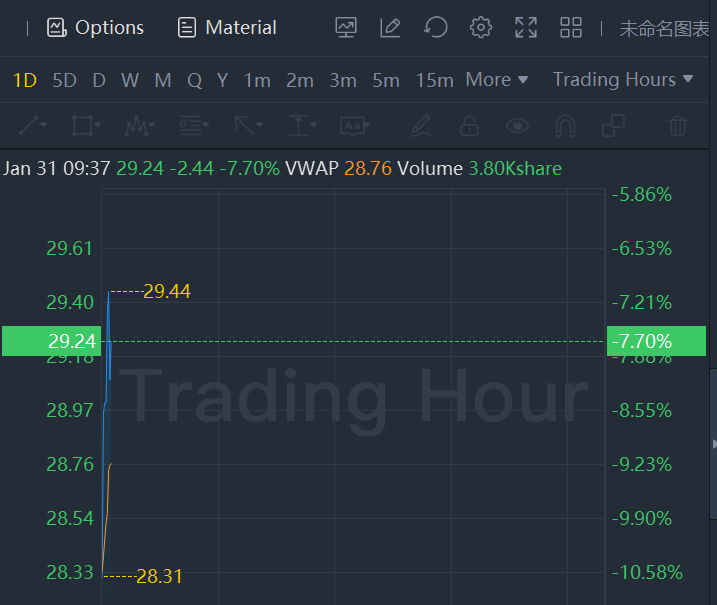 The company and Pfizer Inc. said they discontinued a program testing an experimental treatment for cardiovascular risk reduction and severe hypertriglyceridemia. The companies said that although the drug, vupanorsen, met the primary endpoint in a Phase 2b study, there was concern about its viability as a treatment for those indications. Pfizer had licensed the drug from Ionis in 2019; those rights have now been returned to the company.
The company and Pfizer Inc. said they discontinued a program testing an experimental treatment for cardiovascular risk reduction and severe hypertriglyceridemia. The companies said that although the drug, vupanorsen, met the primary endpoint in a Phase 2b study, there was concern about its viability as a treatment for those indications. Pfizer had licensed the drug from Ionis in 2019; those rights have now been returned to the company.
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.


Comments