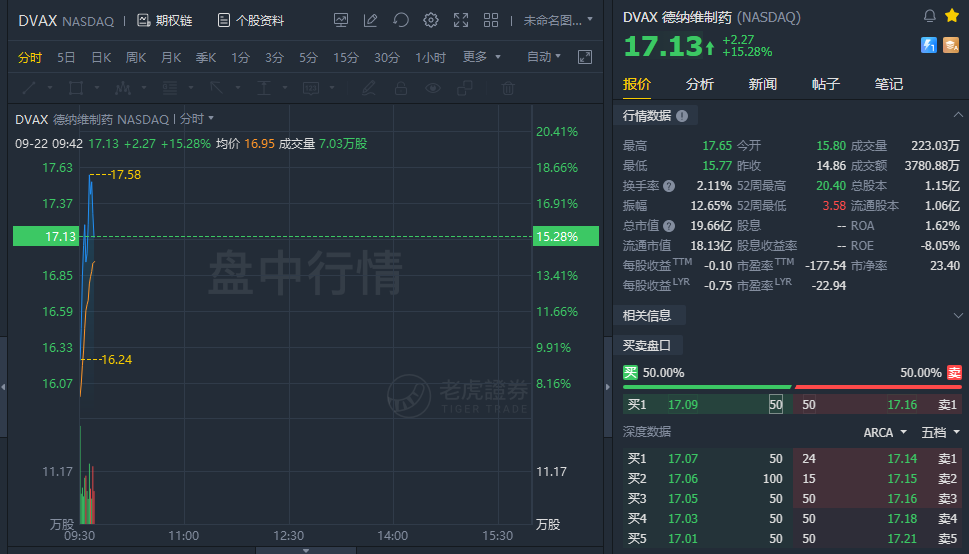
(Sept 22) Dynavax surged over 15% in early trading. Dynavax partner Clover's COVID-19 vaccine shows 79% efficacy against Delta variant.
Clover Biopharmaceuticalsannounces that its adjuvanted protein-based COVID-19 vaccine candidate, SCB-2019 (CpG 1018/Alum) achieved the primary efficacy endpoint and secondary efficacy endpoints in Phase 2/3 SPECTRA trial.
Trial enrolled over 30,000 adult & elderly participants.
Vaccine efficacy was successfully demonstrated where 100% of SARS-CoV-2 strains observed in the efficacy analysis were variants.
79% overall efficacy observed against COVID-19 of any severity caused by globally dominant Delta variant. Efficacy was 92% against Gamma variant and 59% against Mu variant.
Overall efficacy was 67% against COVID-19 of any severity caused by any strain in the study, successfully meeting the primary endpoint of the trial.
Vaccination reduced the risk of symptomatic COVID-19 reinfection caused by any strain by 64.2%.
SCB-2019 demonstrated a favorable safety profile with no significant difference in systemic adverse events compared to placebo.
The vaccine was created by combining SCB-2019 with Dynavax's(NASDAQ:DVAX)CpG 1018 advanced adjuvant and aluminum hydroxide (alum).
Comments