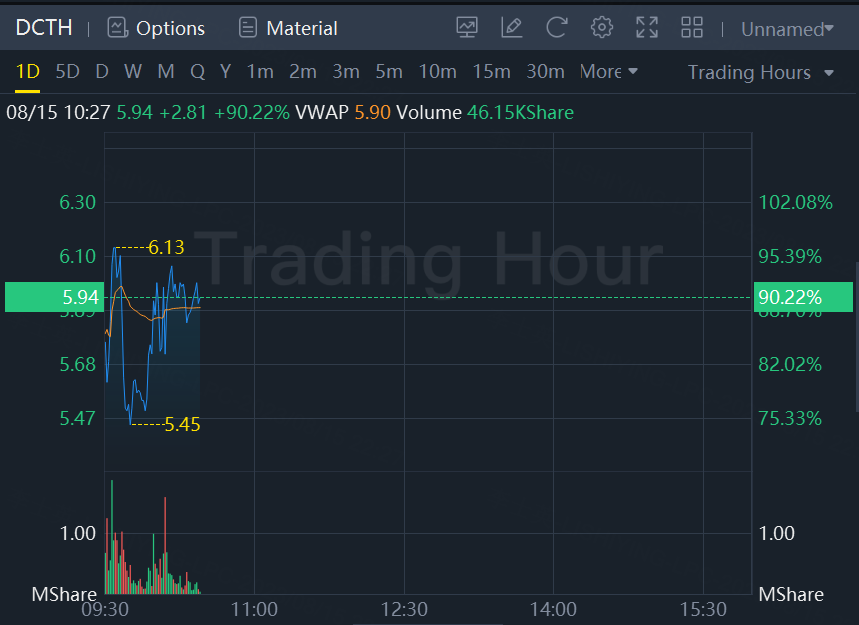
Oncology-focused healthcare equipment maker Delcath Systems (NASDAQ:DCTH) surged 90% in morning trading Tuesday after announcing the FDA approval of its Hepzato Kit device as a treatment for a type of liver-dominant eye cancer.
Accordingly, Hepzato Kit which is designed to deliver chemotherapy Hepzato will be available for as a liver-directed therapy for adults with uveal melanoma ((mUM)), a rare form of cancer that affects melanocytes in the eye.
The New York-based medical equipment maker expects to launch the product in Q4 2023.
The FDA’s decision is based on data from the company’s Phase 3 FOCUS Study which indicated a ~36% objective response rate and 14 months of median duration of response in 91 patients who received Hepzato Kit every 6 – 8 weeks, for up to six treatments.
Hepzato Kit comes with a Boxed Warning related to its safety risks, and the drug’s availability will come under the FDA Risk Evaluation and Mitigation Strategy (REMS) program to address the risks.
Comments