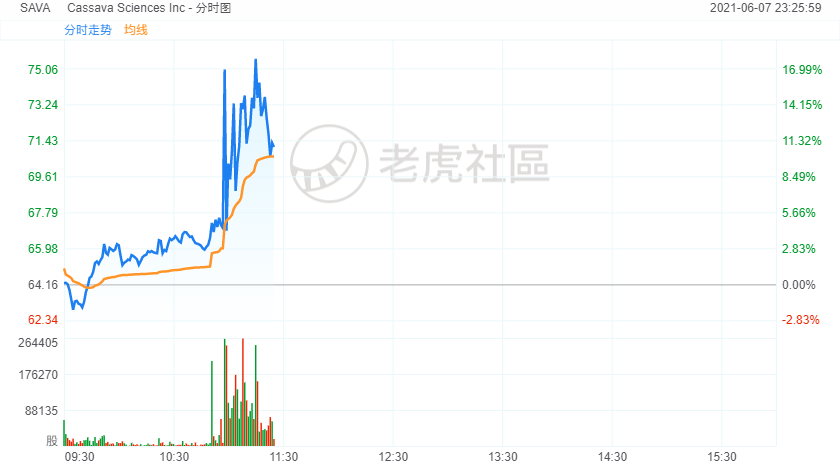
美国食品药品管理局(FDA)批准Biogen的Aduhelm用于治疗阿尔茨海默症,但要求该公司在获批之后继续实施临床试验。FDA表示,Biogen的试验数据高度复杂,存在不确定性。Cassava Sciences(SAVA)维持大约11%的涨幅,一度以75.09美元录得2月4日以来盘中新高,该公司当时宣布老年痴呆症疗法研究取得积极进展,研究结果支持对药品Simufilam进行第三阶段临床试验。
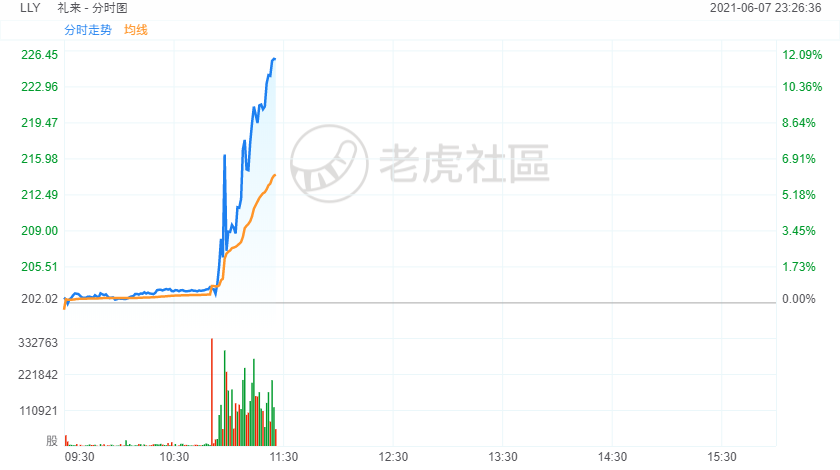
礼来制药涨超11%,刷新盘中历史高位至227美元;礼来同样在开发相同赛道药物多纳单抗(Donanemab)。该公司3月中旬在《新英格兰医学杂志》刊发的阿尔茨海默症试验药品数据显示,其药品前景不错,但并没有投资者所期望的那么喜人。
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.


Comments