Eli Lilly (NYSE:LLY) reported quarterly earnings of $1.25 per share which missed the analyst consensus estimate of $1.69 by 26.04 percent. This is a 33.16 percent decrease over earnings of $1.87 per share from the same period last year. The company reported quarterly sales of $6.49 billion which missed the analyst consensus estimate of $6.70 billion by 3.16 percent. This is a 3.74 percent decrease over sales of $6.74 billion the same period last year.
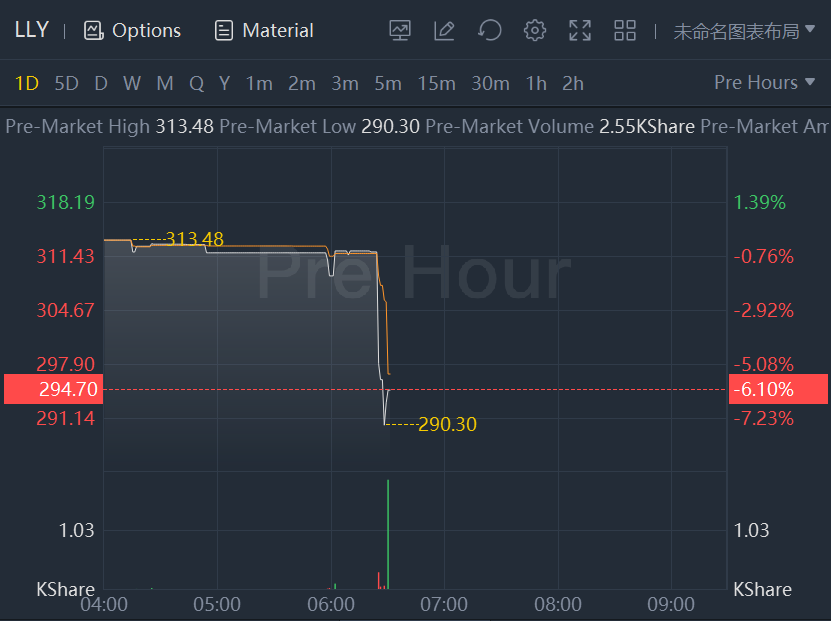
Lilly shares dropped more than 6% after reporting quarterly results.
- Lilly's revenue in Q2 2022 decreased 4%. On a constant currency basis, revenue decreased 1% as lower realized prices and lower Alimta revenue following the entry of generics more than offset volume growth from key growth products. Total revenue grew 6% excluding revenue from Alimta, the sale of the company's rights to Cialis inChinain Q2 2021, and COVID-19 antibodies.
- The company continued to advance its pipeline with theU.S.approval and launch of Mounjaro for type 2 diabetes, approval in theU.S., EU andJapanof Olumiant for alopecia areata, and FDA acceptance with Priority Review designations of donanemab for Alzheimer's disease and pirtobrutinib for mantle cell lymphoma for patients previously treated with a BTK inhibitor, both for review under accelerated approval pathways.
- Key growth products - consisting of Trulicity, Verzenio, Jardiance, Taltz, Retevmo, Mounjaro, Emgality, Olumiant, Tyvyt and Cyramza - grew 20% and represented 67% of revenue in Q2 2022, excluding revenue from COVID-19 antibodies.
- Q2 2022 EPS decreased 31% to$1.05on a reported basis and decreased 32% to$1.25on a non-GAAP basis. Q2 2022 reported and non-GAAP EPS are both inclusive of$0.46of acquired IPR&D and development milestone charges.
- 2022 EPS guidance updated to be in the range of$6.96to$7.11on a reported basis and$7.90to$8.05on a non-GAAP basis, both inclusive of$0.61of acquired IPR&D and development milestone charges.
Eli Lilly and Company today announced its financial results for the second quarter of 2022.
"We had an exciting quarter with the highly anticipatedU.S.launch of Mounjaro, the first of potentially five new medicines we intend to launch by the end of 2023," saidDavid A. Ricks, Lilly's chair and CEO. "We are pleased with the underlying strength of our core business, and we expect our new medicines will add to our growth through the rest of the decade. We are entering a compelling era in our company's history, as we continue our efforts to expand the number of people our medicines can help."
LillySenior Vice President and Chief Financial OfficerAnat Ashkenaziprovided further commentary on the quarter.
"Excluding revenue from the Alimta loss of exclusivity in major markets, the sale of rights to Cialis inChinain the base period, and COVID-19 antibodies, Lilly experienced 6% revenue growth in our core business led by strong performance from key products such as Trulicity, Verzenio and Jardiance," said Ashkenazi. "While we expect that our financial results will continue to be negatively impacted by foreign exchange rates, our revenue guidance for 2022 remains unchanged. Importantly, the inclusion of acquired in-process research and development and development milestone charges in our non-GAAP results will continue to impact comparisons to prior years."
Today, the company is sharing new notable announcements:
- TheU.S. Food and Drug Administration(FDA) accepted, with Priority Review designation, donanemab for Alzheimer's disease for review under the accelerated approval pathway.
- The FDA also accepted, with Priority Review designation, pirtobrutinib for mantle cell lymphoma for patients previously treated with a BTK inhibitor for review under the accelerated approval pathway.
- In collaboration with theU.S.government, Lilly intends to make bebtelovimab commercially available for purchase byU.S.states/territories, hospitals and a broad set of other providers through a sole distributor beginning the week ofAug. 15, which is prior to the anticipated depletion of theU.S.government's currently available supply.
Lilly shared numerous updates recently on key regulatory, clinical, business development and other events, including:
- Mounjaro®(tirzepatide) for adults with type 2 diabetes was approved by the FDA and received a positive opinion from theEuropean Medicines Agency'sCommittee for Medicinal Products for Human Use.
- Lilly and Incyte's Olumiant®(baricitinib) for adults with severe alopecia areata received approval in theU.S., EU andJapan.
- The FDA approved Olumiant for the treatment of certain hospitalized patients with COVID-19.
- The company announced positive topline results from Phase 3 clinical trials of lebrikizumab for the treatment of patients with moderate-to-severe atopic dermatitis.
- Lilly supplied additional doses of bebtelovimab to theU.S.government in an ongoing effort to provide COVID-19 treatment options for patients.
- The company announced plans to invest$2.1 billionin two newIndianamanufacturing sites.
Comments