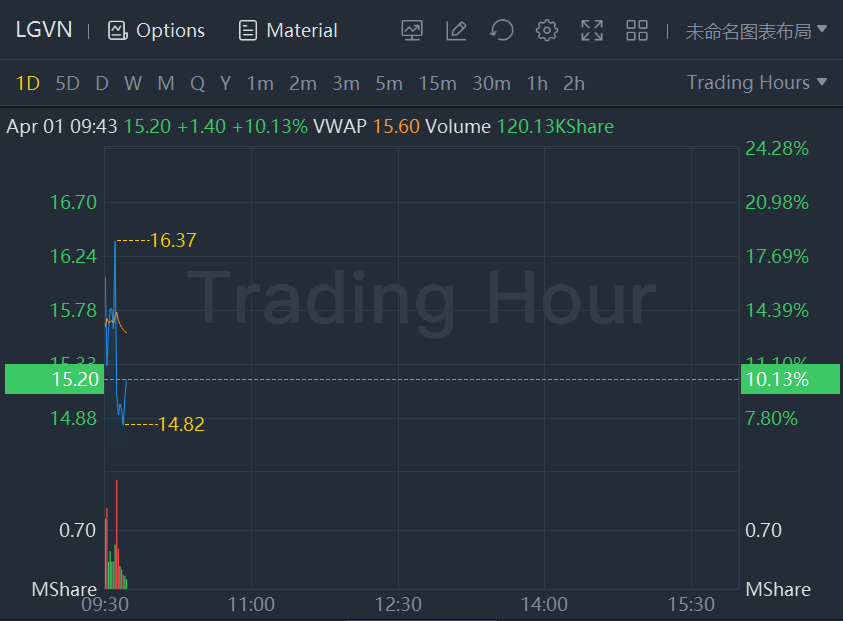
Longeveron shares jumped another 10% in morning trading. The stock soared 105% yesterday.
Clinical-stage biotech announcingthe peer-reviewed publication of results from a Phase 1 trial for its Alzheimer’s disease candidate in Lomecel-B on Thursday.
The data were published in the Journal of the Alzheimer’s Association with the title “Results and Insights from a Phase 1 Clinical Trial of Lomecel-B for Alzheimer’s disease,” the company said.
Lomecel-B, Longeveron’s (LGVN) lead candidate, is a cell-based therapy product derived from the bone marrow of young, healthy adult donors.
The 33-patient Phase 1 trial, designed to evaluate its effect in patients with mild Alzheimer’s disease, had previously met the primary endpoint indicating it was well-tolerated among patients.
“We are pleased and encouraged by the publication of our study in this high-impact journal,” Chief Executive Geoff Green noted.
In January, Longeveron (LGVN) announced the initiation of Phase 2a clinical trial for the candidate in Alzheimer’s.
Comments