Black Diamond Therapeutics shares surged nearly 24% in premarket trading.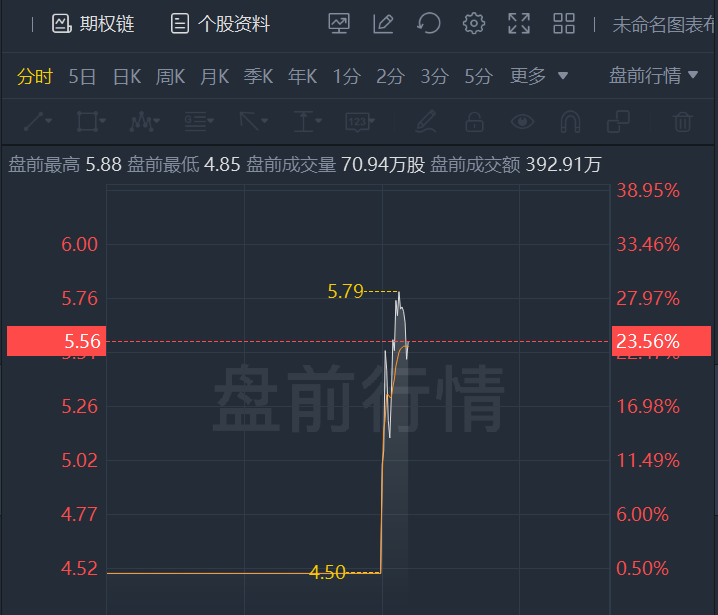 Black Diamond Therapeutics, Inc., a precision oncology medicine company pioneering the discovery and development of MasterKey therapies, today announced that the U.S. Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for its MasterKey inhibitor BDTX-1535, an irreversible, mutant selective, brain-penetrant inhibitor of oncogenic mutations of epidermal growth factor receptor (EGFR) expressed in glioblastoma multiforme (GBM) and intrinsic and acquired resistance EGFR mutations in non-small cell lung cancer (NSCLC). The Company expects to initiate the Phase 1 study of BDTX-1535 in the first quarter of 2022 and expects to provide a clinical update in the second half of 2023.
Black Diamond Therapeutics, Inc., a precision oncology medicine company pioneering the discovery and development of MasterKey therapies, today announced that the U.S. Food and Drug Administration (FDA) has cleared an investigational new drug (IND) application for its MasterKey inhibitor BDTX-1535, an irreversible, mutant selective, brain-penetrant inhibitor of oncogenic mutations of epidermal growth factor receptor (EGFR) expressed in glioblastoma multiforme (GBM) and intrinsic and acquired resistance EGFR mutations in non-small cell lung cancer (NSCLC). The Company expects to initiate the Phase 1 study of BDTX-1535 in the first quarter of 2022 and expects to provide a clinical update in the second half of 2023.
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.


Comments