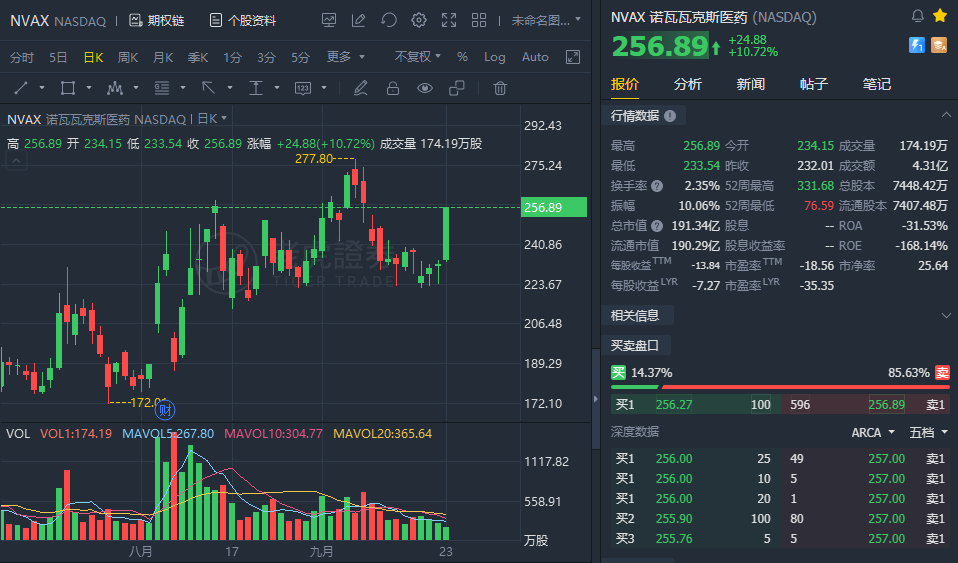
(Sept 23) Novavax surged over 10% in early trading. Novavax Announce Submission to World Health Organization for Emergency Use Listing of Novavax' COVID-19 Vaccine.
Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious diseases, with its partner, Serum Institute of India Pvt. Ltd. (SII), today announced a regulatory submission to the World Health Organization (WHO) for emergency use listing (EUL) of Novavax' recombinant nanoparticle protein-based COVID-19 vaccine candidate with Matrix-M™ adjuvant. The submission to WHO is based on the companies' previous regulatory submission to the Drugs Controller General ofIndia(DCGI).
"Today's submission of our protein-based COVID-19 vaccine to WHO for emergency use listing is a significant step on the path to accelerating access and more equitable distribution to countries in great need around the world," saidStanley C. Erck, President and Chief Executive Officer, Novavax. "It represents another major milestone in Novavax' transformation into a commercial global vaccine company and reinforces the value of global collaboration and need for multiple approaches to help control the pandemic."
The grant of EUL by the WHO is a prerequisite for exports to numerous countries participating in the COVAX Facility, which was established to allocate and distribute vaccines equitably to participating countries and economies. In addition to the submission for WHO EUL, SII and Novavax last month completed the submission of modules required by regulatory agencies inIndia,Indonesiaandthe Philippinesfor the initiation of review of the vaccine, including preclinical, clinical, and chemistry, manufacturing and controls (CMC) data.
Comments