Aldeyra Therapeutics Inc reported topline data from Phase 3 TRANQUILITY Trial of reproxalap ophthalmic solution for dry eye disease.
- The trial failed to meet the primary endpoint of ocular redness, but it did achieve statistical significance for the dry eye disease sign of the Schirmer test, a secondary endpoint.
- Statistical significance was also achieved for the post-hoc assessment of Schirmer test responders of over 10 mm.
- The primary endpoint of the upcoming Phase 3 TRANQUILITY-2 trial has been modified such that the endpoint will have been met if either the Schirmer test or ocular redness demonstrates statistical significance.
- In addition, target enrollment in TRANQUILITY-2 has been increased from 300 to up to 400 patients. The company expects topline results in mid-2022.
- The company intends to file the marketing application for dry eye disease indication in mid-2022.
- Application for allergic conjunctivitis, another proposed indication, is expected to occur after the dry eye disease submission and following completion of an additional allergen chamber trial requested by the FDA.
- No safety signals were observed, and reproxalap was well tolerated; there were no treatment-related discontinuations or moderate or severe adverse events.
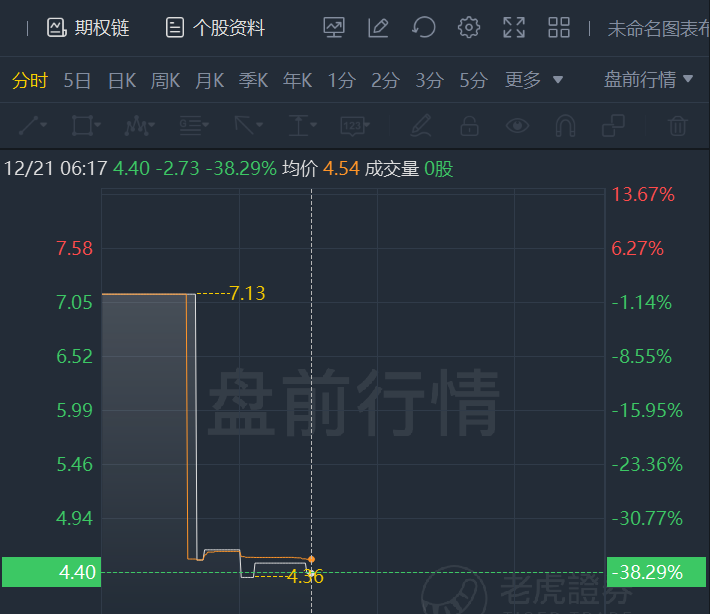
- Price Action: ALDX shares are down 38.29% at $4.4 during the premarket session on the last check Tuesday.
Comments