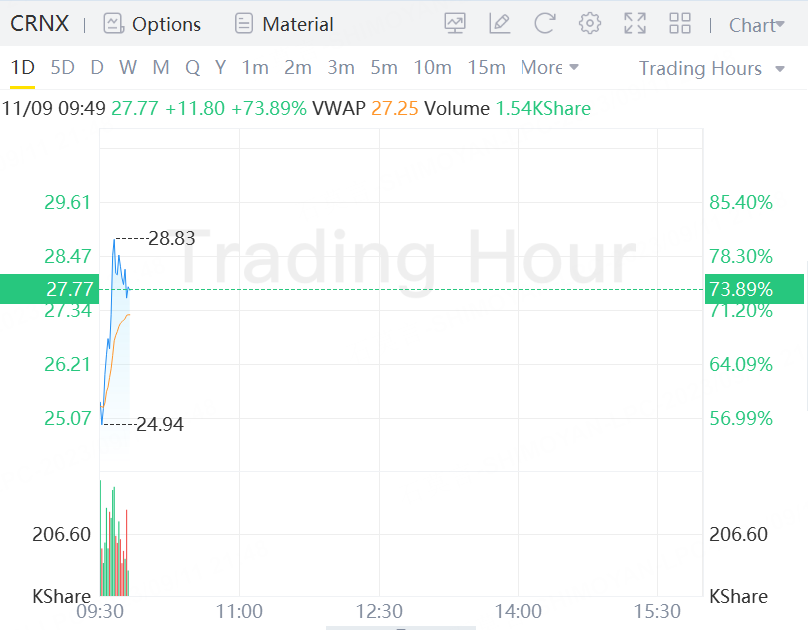
Crinetics Pharmaceuticals added 73.89% pre-market Monday after announcing that its lead candidate, paltusotine, achieved the primary endpoint and all secondary endpoints in a Phase 3 trial for patients with the rare disease acromegaly.
The PATHFNDR-1 study enrolled 58 adults with acromegaly, characterized by excessive growth hormone secretion. After switching from standard-of-care, they received once-daily oral paltusotine or a placebo for 36 weeks .
The trial met statistical significance (p<0.0001) for the primary endpoint as 83% of patients on paltusotine maintained an insulin-like growth factor 1 (IGF-1) level ≤ 1.0 times the upper limit of normal, compared to 4% on placebo.
All secondary endpoints of the trial also achieved statistical significance. Regarding safety, the proportion of patients with at least one treatment-emergent adverse event (TEAE) stood at 80% and 100% for the on-drug and off-drug arms, respectively.
Crinetics intends to seek U.S. regulatory approval for paltusotine in 2024, subject to positive results from PATHFNDR-2, an ongoing Phase 3 study for the drug targeted at untreated acromegaly patients. That trial is fully enrolled and is expected to generate early data in Q1 2024.
Comments