Regeneron Pharmaceuticals reported a 72.5% fall in quarterly profit on Wednesday, hurt by lacklustre sales of its COVID-19 antibody cocktail after the U.S. health regulator decided to limit its use earlier this year.
The drugmaker's net profit fell to $852 million, or $7.47 per share, in the second quarter ended June 30, compared with $3.1 billion, or $27.97 per share, a year earlier.
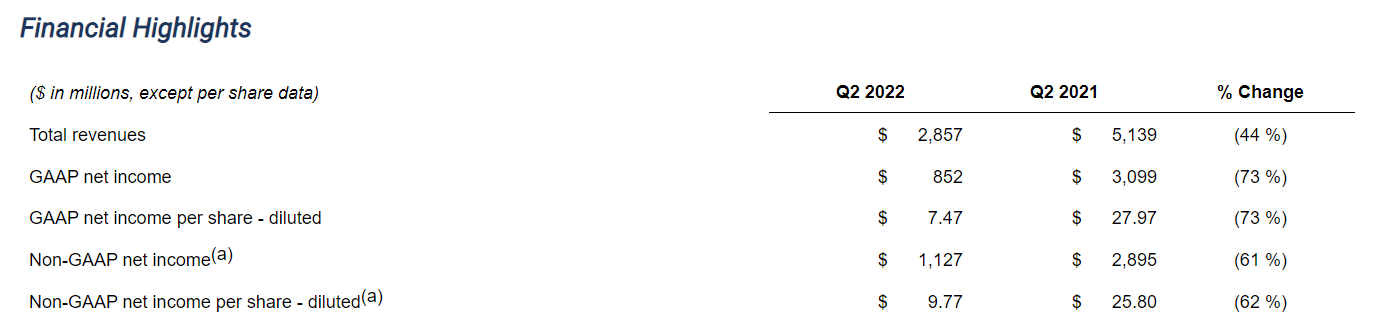
- Second quarter 2022 revenues decreased 44% to$2.86 billionversus second quarter 2021; excluding REGEN-COV®(a)(b), revenues increased 20%
- Second quarter 2022 EYLEA®U.S.net sales increased 14% versus second quarter 2021 to a record$1.62 billion
- Second quarter 2022 Dupixent®global net sales(c)(recorded by Sanofi) increased 40% to$2.09 billionversus second quarter 2021
- Second quarter 2022 GAAP diluted EPS of$7.47; non-GAAP diluted EPS(a)of$9.77including unfavorable$1.71impact from acquired IPR&D charges
- FDA approved Dupixent for atopic dermatitis in children aged 6 months to 5 years and eosinophilic esophagitis in adults and adolescents; FDA priority review granted for prurigo nodularis
- Encouraging preliminary anti-tumor activity observed for novel investigational PSMAxCD28 costimulatory bispecific antibody in combination with Libtayo®in advanced metastatic castration-resistant prostate cancer
- Strengthened commitment to oncology through purchase of Sanofi's stake in Libtayo and acquisition ofCheckmate Pharmaceuticals
Regeneron Pharmaceuticals, Inc.(NASDAQ: REGN) today announced financial results for the second quarter of 2022 and provided a business update.
"The second quarter of 2022 was distinguished by record net product sales of EYLEA, Dupixent, and Libtayo, as well as multiple regulatory achievements for Dupixent, includingU.S.approvals for atopic dermatitis among very young patients and for eosinophilic esophagitis in adults and adolescents, as well as European approval for pediatric asthma," saidLeonard S. Schleifer, M.D., Ph.D., President and Chief Executive Officer of Regeneron. "In addition, we have continued to strengthen our oncology franchise, including through the purchase of worldwide rights to Libtayo as well as encouraging but preliminary anti-tumor activity observed at higher doses of our novel PSMAxCD28 costimulatory bispecific in combination with Libtayo for advanced metastatic castration-resistant prostate cancer."
"We are pleased with our second quarter 2022 financial performance, including 20% revenue growth when excluding contributions fromREGEN-COV. This demonstrates the continued strength of our core business," saidRobert E. Landry, Executive Vice President, Finance and Chief Financial Officer of Regeneron. "Additionally, we updated our full-year 2022 financial guidance primarily to reflect the recently completed acquisition of Libtayo global rights from Sanofi, a transaction that we believe will deliver significant shareholder value over time. In the second half of 2022, we look forward to advancing our pipeline with important clinical data readouts in oncology and ophthalmology as well as continued commercial execution and prudent capital allocation to drive value creation for shareholders."
Business Highlights
Key Pipeline ProgressRegeneron has approximately 35 product candidates in clinical development, including a number of marketed products for which it is investigating additional indications. Updates from the clinical pipeline include:
Dupixent® (dupilumab)
- InJune 2022, theU.S. Food and Drug Administration(FDA) approved Dupixent as the first biologic medicine for children aged 6 months to 5 years with moderate-to-severe atopic dermatitis.
- InMay 2022, the FDA approved Dupixent for adults and adolescents aged 12 years and older with eosinophilic esophagitis (EoE).
- InApril 2022, theEuropean Commission(EC) approved Dupixent for the treatment of severe asthma in children aged 6 to 11 years.
- The Company and Sanofi announced positive results from a Phase 3 trial in children aged 1 to 11 years with EoE. The trial met its primary endpoint of histological disease remission at 16 weeks with both higher and lower dose weight-tiered regimens.
- The FDA accepted for priority review the supplemental Biologics License Application (sBLA) for Dupixent for adults with prurigo nodularis, with a target action date ofSeptember 30, 2022. Regulatory applications have also been submitted in theEuropean Union(EU) andJapan.
EYLEA® (aflibercept) Injection
- The FDA accepted for review the sBLA for EYLEA for an every-16-weeks dosing regimen in patients with diabetic retinopathy (DR), with a target action date ofFebruary 28, 2023.
REGN5678, a PSMAxCD28 costimulatory bispecific antibody
- Reported preliminary, first-in-human data in combination with Libtayo®in patients with advanced metastatic castration-resistant prostate cancer.
Antibodies to SARS-CoV-2 virus
- The Company is continuing to progress investigational "next generation" antibodies that are active against multiple variants including those of Omicron-lineage.
REGN5381, an agonist antibody toNPR1
- A Phase 2 study in heart failure was initiated.
Corporate and Business Development Updates
- InMay 2022, the Company completed its acquisition ofCheckmate Pharmaceuticals, Inc.for a total equity value of approximately$250 million. In connection with the acquisition, the Company obtained the rights to vidutolimod (immune activator targeting TLR9), which is in clinical development for oncology.
- EffectiveJuly 1, 2022, the Company obtained the exclusive right to develop, commercialize, and manufacture Libtayo worldwide under an Amended and Restated Immuno-oncology License and Collaboration Agreement with Sanofi. Under the terms of the agreement, the Company made a$900 millionup-front payment, and Sanofi is eligible to receive a$100 millionregulatory milestone and up to an aggregate of$100 millionin sales-based milestones upon achieving certain amounts of worldwide net product sales of Libtayo through 2023. The Company will also pay Sanofi a royalty on net product sales of Libtayo.
- Also effectiveJuly 1, 2022, the Company will increase from 10% to 20% the share of its profits that are paid to Sanofi in connection with the development balance reimbursement under the antibody collaboration.
Comments