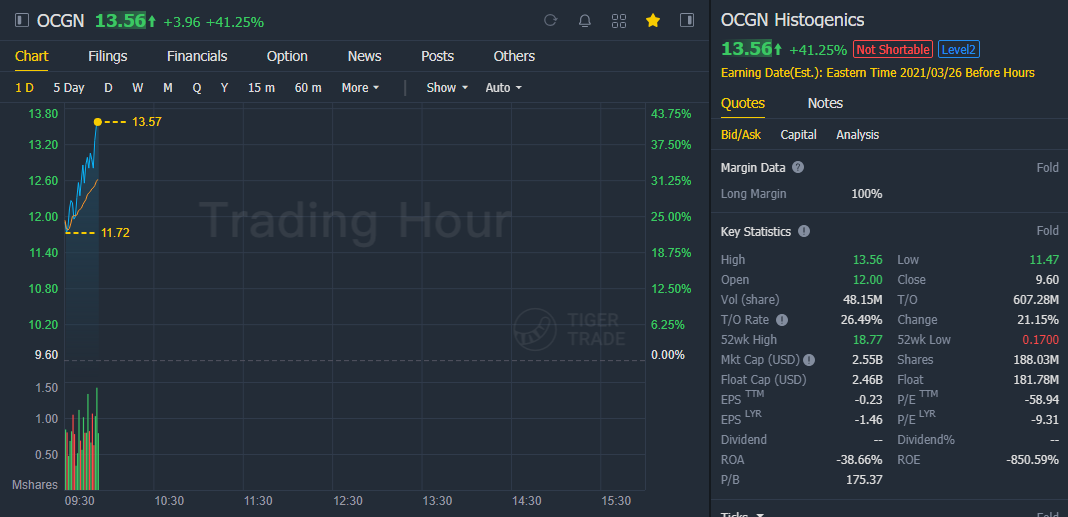
(March 3) Shares of Histogenics soared 41.0%, after the biopharmaceutical company said its co-development partner Bharat Biotech released an interim analysis of its Phase 3 trial of its COVID-19 vaccine candidate, COVAXIN, which demonstrated efficacy of 81%.
Bharat's Phase 3 trial in India enrolled 25,800 participants aged 18 to 91, and the first interim analysis is based on 43 cases. A review of the safety database showed severe, serious and medically attended adverse events occurred at low levels and balanced between vaccine and placebo groups.
"These results, which in part suggest significant immunogenicity against the rapidly emerging UK variant, represent an additional step towards outlining the regulatory pathway for EUA and approval in the United States," said Ocugen Chief Executive Shankar Musunuri.
"COVAXIN, a whole virion based vaccine candidate, is designed to fill a significant unmet need in our national arsenal of vaccines against COVID-19." Ocugen's stock has skyrocketed 3,071.5% over the past three months, while the iShares Nasdaq Biotechnology ETF has tacked on 6.8% and the S&P 500 has gained 5.6%.
Comments