U.S. Calls For Pause On Johnson & Johnson Vaccine After Clotting Cases.
KEY POINTS
- The FDA said it is asking states to temporarily halt using J&J’s Covid-19 vaccine after six people in the U.S. developed a rare blood clotting disorder.
- The FDA said the recommendation is “out of an abundance of caution.”
- All six cases occurred in women between the ages of 18 and 48, with symptoms developing six to 13 days after they received the shot.
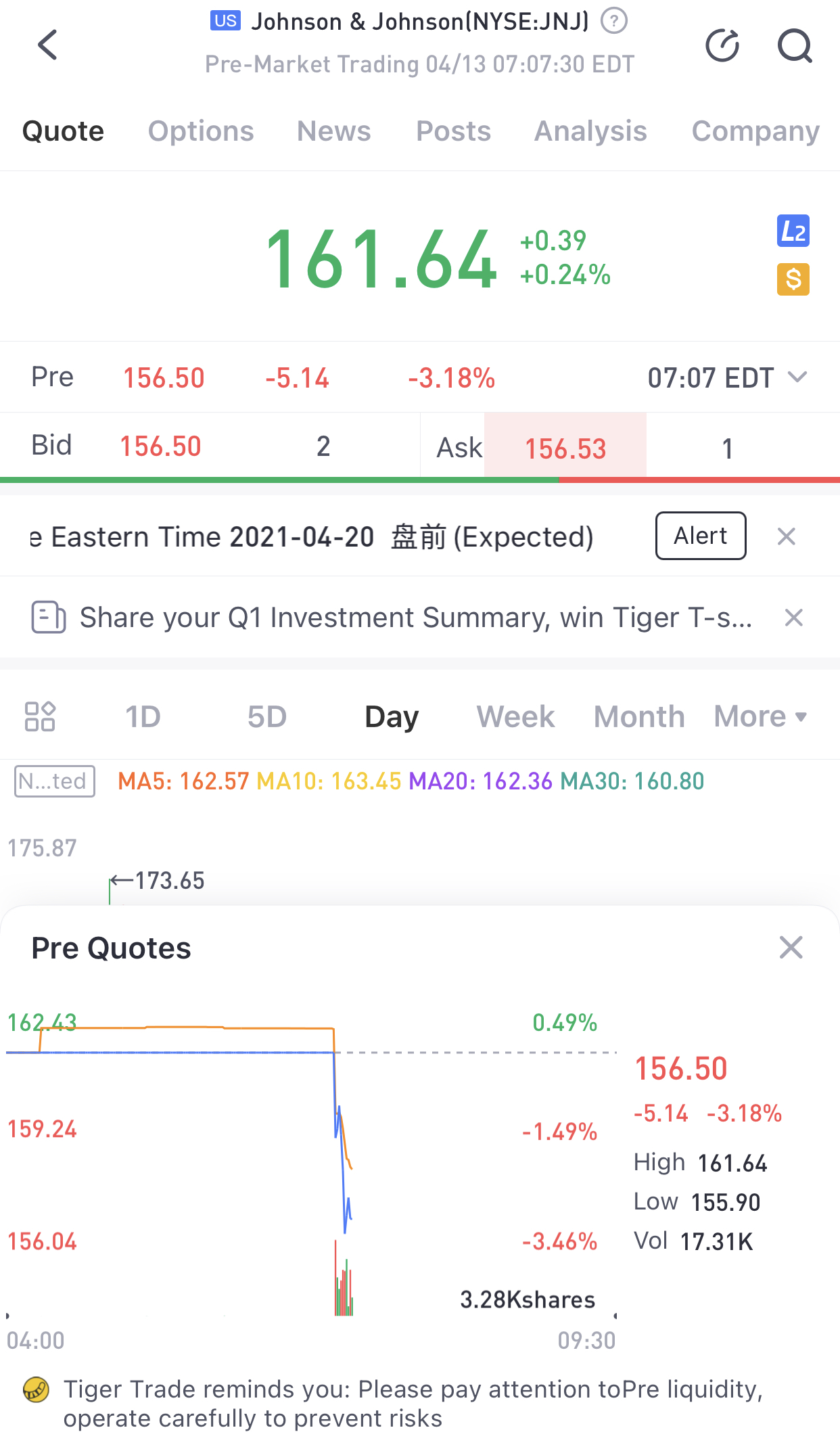
The Food and Drug Administration said Tuesday it is asking states to temporarily halt usingJohnson & Johnson’s Covid-19 vaccine after six people in the U.S. developed a rare blood clotting disorder.
The FDA said the recommendation is “out of an abundance of caution.”
“Right now, these adverse events appear to be extremely rare,” the FDA said in a joint statement with the Centers for Disease Control and Prevention. “COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of health problems following COVID-19 vaccination very seriously.”

All six cases occurred in women between the ages of 18 and 48, with symptoms developing six to 13 days after they received the shot. Doctors typically treat that type of blood clot with heparin but health regulators noted that could be dangerous in this case and recommended a different treatment.
J&J said in a statement that “no clear causal relationship” has been identified between the blood clots and the vaccine, adding it is working closely with regulators to assess the data.
People who receive the vaccine and “develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider,” the FDA and CDC said.
Shares of J&J were down more than 3% in premarket trading Tuesday.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on Wednesday to further review the cases, federal health regulators said Tuesday. The FDA is also investigating the cases.
J&J’s Covid-19 vaccine, like Pfizer’s and Moderna’s shots, received emergency use authorization from the FDA to start distributing the doses across the U.S. An EUA grants conditional clearance based on two months of safety data, pending full approval which usually requires at least six months of data.
When J&J submitted its Covid vaccine data to the FDA in February, no specific concerns were identified when analyzed by age, race and comorbidities, according to the agency. The FDA said at the time the most common side effects reported were headache and fatigue, followed by muscle aches, nausea and fever.
The New York Timesfirst reportedthe news.
It’s unclear how the pause will impact J&J’s goal to deliver 100 million doses to the U.S. by the end of May. The company has already been plagued by manufacturing issues of its vaccine.
Last week, Europe’s medicines regulatorsaid it found a possible linkbetween the coronavirus vaccine developed by AstraZeneca and the University of Oxford and rare blood clotting issues. AstraZeneca has not received authorization for use in the U.S.
Emer Cooke, executive director of the European Medicines Agency, said in a televised news conference last week that unusual blood clotting with low blood platelets would be added as a “very rare” side effect to the vaccine’s product information, along with a slew of other possible adverse reactions.
Comments