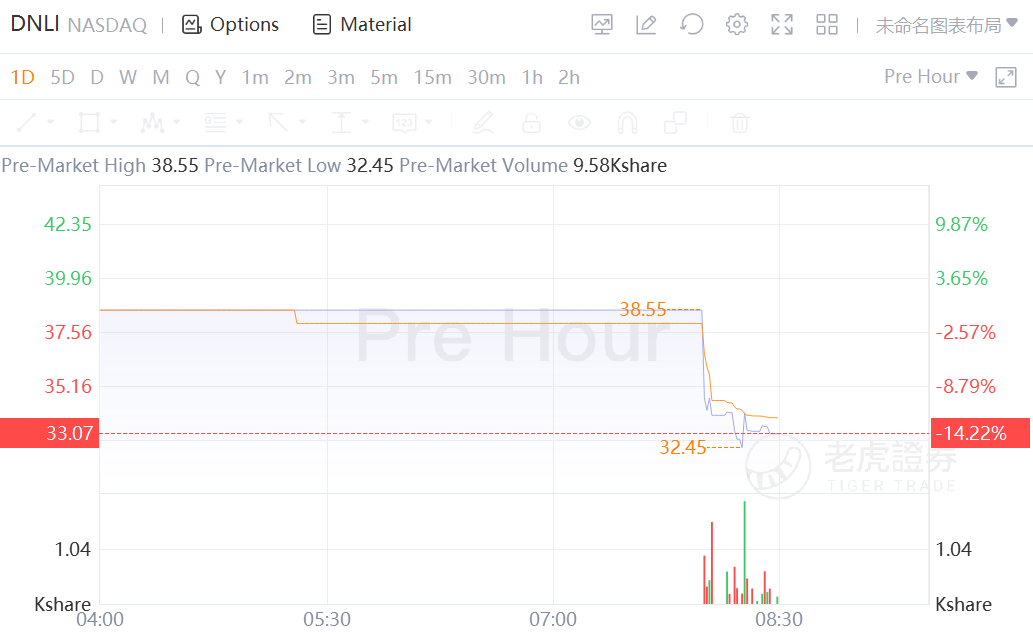
Denali Therapeutics fell 14% in the pre-market after announcing a clinical hold imposed by the FDA for the company’s experimental therapy for Alzheimer’s disease, DNL919.
On Wednesday after the close, the regulator has informed the company via email that the DNL919 (ATV:TREM2) Investigational New Drug (IND) application had been placed on clinical hold.
An official clinical hold letter will be issued to Denali (DNLI) in this regard in about 30 days, the federal agency has added. The company plans to announce further updates on this development subject to its ongoing discussions with the regulator.
According to the therapeutic pipeline of the company, DNL919 (ATV:TREM2) is part of its Alzheimer’s program. Early this week, Denali (DNLI) said that subject to the FDA acceptance of its IND application, the company would begin first-in-human clinical trials for DNL919 in H1 2022.
Comments