Novavax Shares Fall 3% in Morning Trading.
Novavax Won't Get Authorization Until the FDA Reviews Manufacturing Changes.
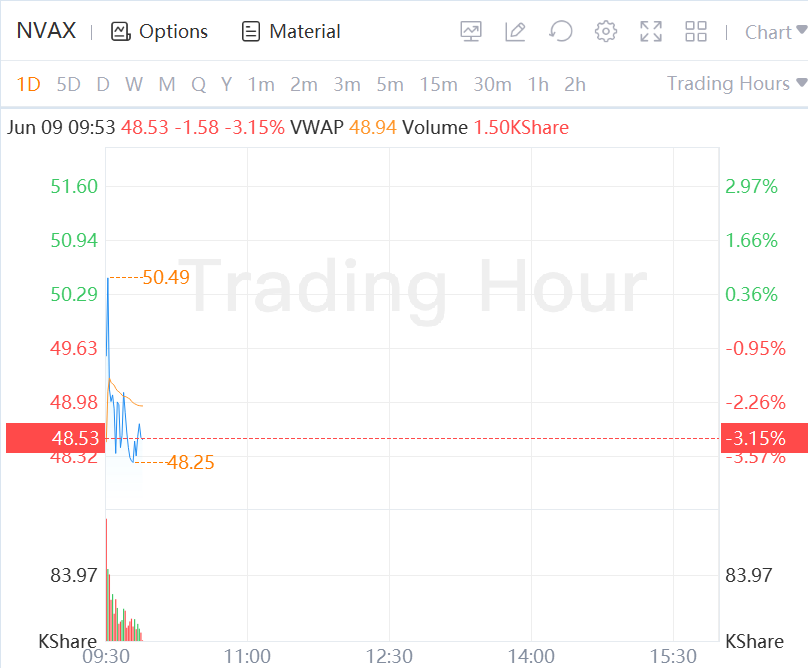
Not so fast.
Early Thursday, CNBC reported that the FDA will need to review changes to the company's manufacturing process before issuing an emergency-use authorization. Novavax (ticker: NVAX) informed the FDA of those changes on June 3, CNBC said.
That could take weeks, or more. In a note on Wednesday, J.P. Morgan analyst Eric Joseph wrote that he expects a decision on an emergency-use authorization for the vaccine would come in the middle of July. Novavax told Joseph that it expected to complete its submission of data on the manufacturing changes and receive an emergency-use authorization within "weeks."
Novavax shares were down 6.2% in premarket trading Thursday, to $47. That brings the stock below the $47.54 price point at which it closed on Monday, before the FDA's outside advisors voted in favor of the proposition that the vaccine's benefits outweighed its risks.
At that meeting, the director of the FDA's Center for Biologics Evaluation and Research, Dr. Peter Marks, made clear that he sees a need for Novavax's vaccine, which unlike the Pfizer $(PFE)$ and Moderna $(MRNA)$ Covid-19 vaccines, uses a well-established, protein-based approach.
"Having a protein-based alternative may be more comfortable for some, in terms of their acceptance of vaccine," Marks said at the meeting. "We do have a problem with vaccine uptake that is very serious in the United States, and anything that we can do to get people more comfortable to be able to accept these potentially lifesaving medical products is something we feel we are compelled to do."
Still, Novavax's manufacturing problems have been a longstanding issue, blamed for the delay in bringing the vaccine to market. Asked at the Tuesday advisory committee hearing why Novavax had been so slow at completing its submission for an emergency-use authorization, the company's chief medical officer, Dr. Filip Dubovsky, said that developing and deploying the tests to guarantee the consistency of the vaccine was what "took the longest time."
Novavax said at the committee meeting that doses destined for the U.S. would be made by the Serum Institute of India, the largest vaccine manufacturer in the world by volume. In its briefing documents and presentations, the FDA said that it wouldn't consider some tests run by Novavax of its Covid-19 vaccine, due to changes in the manufacturing process and testing that had occurred over time.
Novavax shares were down 65% so far this year as of the close of trading on Wednesday, and 75.6% over the past 12 months. In his Wednesday note, J.P. Morgan's Joseph wrote that Novavax says it can make two billion doses this year.
"We see upcoming regulatory submissions / label expansion and clinical readouts (Omicron variant) as key to NVAX achieving its top-line guidance of $4-5B for 2022," Joseph wrote.
Comments