G1 Therapeutics Inc. said it has decided to discontinue its Phase 3 trial, called Preserve 1, that tested trilaciclib administered along with triplet therapy with Folfoxiri and bevacizumab in patients with metastatic colorectal cancer.
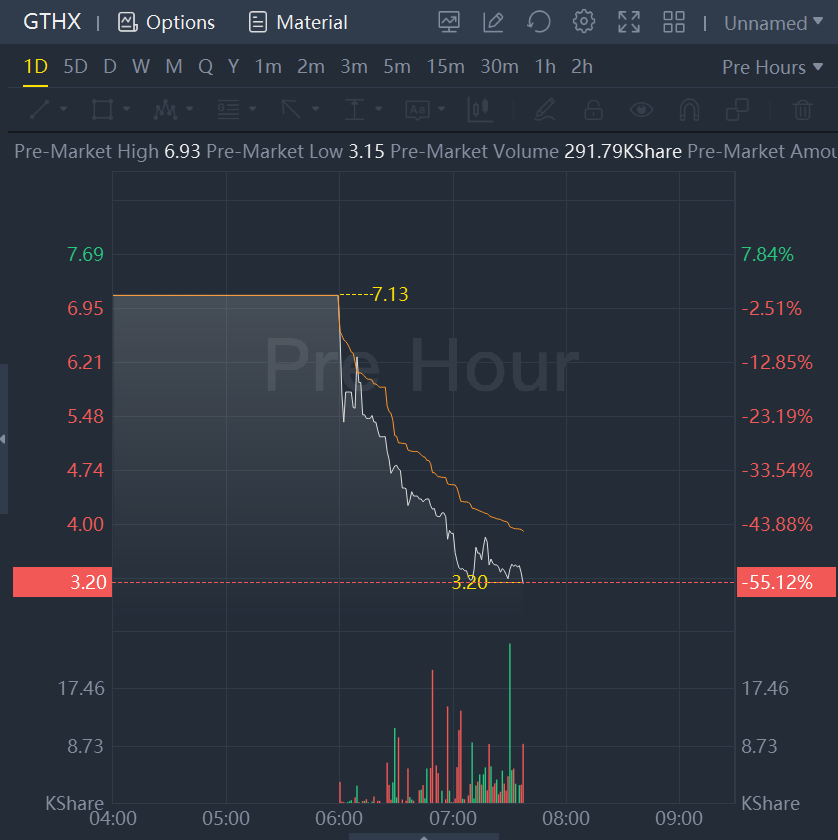
Shares fell 55% to $3.2 in premarket trading.
The company said the trial achieved its co-primary endpoints showing clinically meaningful reductions in severe neutropenia during induction, as well as in the average duration of severe neutropenia. Patients treated with trilaciclib also showed a reduction in the rate of chemotherapy-induced diarrhea, the company said.
However, early anti-tumor efficacy data favored patients in the placebo arm of the trial, the company said.
"All of us at G1 are disappointed in this surprising outcome for patients with CRC, but we remain committed to the potential of trilaciclib to impact the lives of many cancer patients in other indications," G1 Chief Executive Jack Bailey said. "We are increasingly encouraged by the real-world performance of trilaciclib in patients with extensive stage small cell lung cancer and look forward to upcoming readouts in our other ongoing trials."
Comments