Shares of SpringWorks Therapeutics up 14.4% to about $24 in premarket trading.
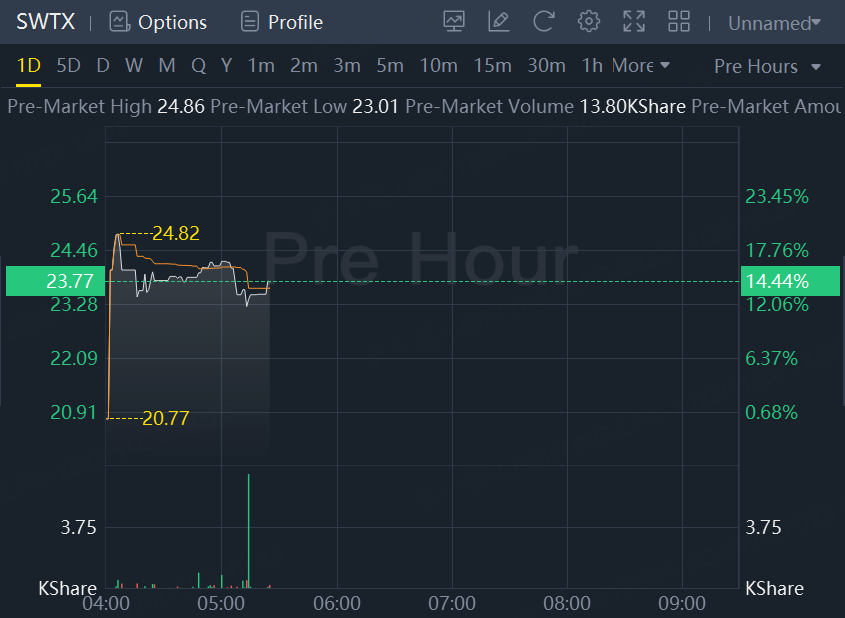
After market close on Monday, U.S. health regulator approved co's drug, Ogsiveo, for treating adult patients with desmoid tumors, making it the first approved treatment for this type of non-cancerous soft-tissue growth.
The monotherapy nirogacestat, branded as Ogsiveo, to be available in the United States within 10 days of approval - CEO.
Desmoid tumors are rare, abnormal, non-cancerous growths that occur in connective tissues and are associated with a high rate of recurrence.
As of last close, stock down 20.2% YTD.
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.

Comments