PK Study Successfully Demonstrates that Fingerprint Sweat Provides Reliable Sample for Drug Detection
FDA 510(k) Clearance Would Enable the Introduction of INBS’ Technology to the US in 2025
NEW YORK, Nov. 13, 2024 (GLOBE NEWSWIRE) -- Intelligent Bio Solutions Inc. (Nasdaq: INBS) ("INBS" or the "Company"), a medical technology company delivering intelligent, rapid, non-invasive testing solutions, today announced strong initial results from its Pharmacokinetic (PK) study required for an FDA 510(k) submission. The data from the PK study shows that fingerprint sweat mimics the rate and extent of codeine in blood and saliva. The study successfully demonstrated that fingerprint sweat provides a reliable sample matrix for drug detection, showing quantitative PK data closely aligned to blood, based on statistical comparisons made at the 95% confidence level.
"The close correlation of PK parameters in fingerprint sweat and blood highlights its robustness as a sampling approach. While independent data has consistently supported this, our clinical study further reinforces these findings,” said Harry Simeonidis, President and CEO of Intelligent Bio Solutions. “We are very pleased with the PK study results. This data highlights the potential for our technology to achieve widespread adoption in safety-critical industries and beyond, marking a significant achievement as we advance toward FDA clearance in the United States."
FDA 510(k) clearance would enable INBS to introduce its innovative drug screening technology to the US market in 2025. The PK study results and other clinical data from the Company's clinical study plan will be submitted as part of the Company's 510(k) submission, expected in the fourth calendar quarter of this year. INBS is well-positioned to meet the increasing demand for drug testing in the United States, particularly in safety-critical industries like construction, mining, and transportation, as well as in law enforcement, drug rehabilitation, and forensic sectors.
Results: Average Codeine Values in Whole Blood, Oral Fluid and Fingerprint Sweat1
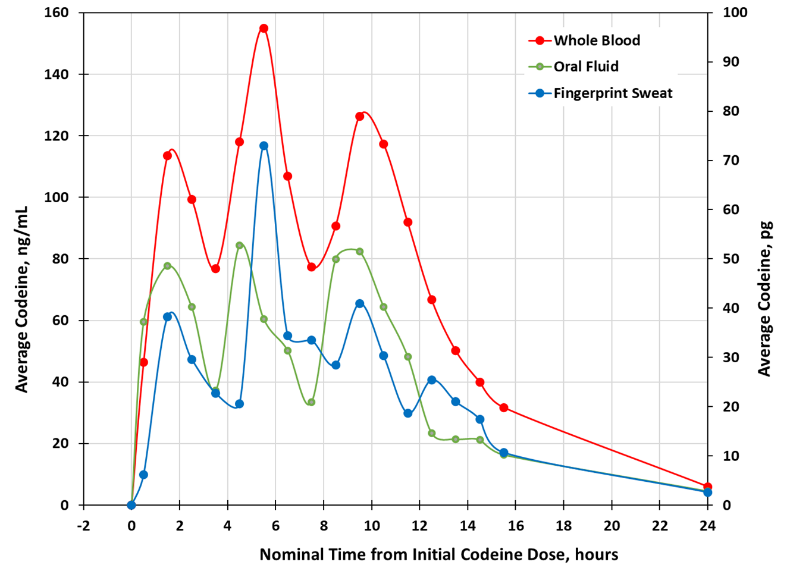
The study results show that fingerprint sweat is a strong indicator of codeine ingestion. They further validate the ability of INBS' method to provide rapid and reliable drug screening through a simple fingerprint sweat sample without invasive procedures.
The PK study, conducted in partnership with Cliantha Research, required a minimum of 36 subjects. The Company successfully recruited 39 healthy adult subjects from diverse backgrounds, including varying genders, ages, and ethnicities. The study compared the levels of opiates detected in fingerprint sweat with those found in blood, oral fluid, and urine samples following the medically supervised administration of codeine. All fingerprint sweat specimens collected using INBS' Intelligent Fingerprinting Drug Screening System, were analyzed by a validated, traceable liquid chromatography/tandem mass spectrometry (LC-MS/MS) method, widely accepted as the gold standard for such studies.
About Intelligent Bio Solutions
Intelligent Bio Solutions Inc. (NASDAQ: INBS) is a medical technology company delivering innovative, rapid, non-invasive testing solutions. The Company believes that its Intelligent Fingerprinting Drug Screening System will revolutionize portable testing through fingerprint sweat analysis, which has the potential for broader applications in additional fields. Designed as a hygienic and cost-effective system, the test screens for the recent use of drugs commonly found in the workplace, including opiates, cocaine, methamphetamine, and cannabis. With sample collection in seconds and results in under ten minutes, this technology would be a valuable tool for employers in safety-critical industries. The Company’s current customer segments outside the US include construction, manufacturing and engineering, transport and logistics firms, drug treatment organizations, and coroners.
For more information, visit: http://www.ibs.inc/
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, Intelligent Bio Solutions Inc.'s ability to successfully develop and commercialize its drug and diagnostic tests, realize commercial benefit from its partnerships and collaborations, and secure regulatory approvals, among others. Although Intelligent Bio Solutions Inc. believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Intelligent Bio Solutions Inc. has attempted to identify forward-looking statements by terminology, including "believes," "estimates," "anticipates," "expects," "plans," "projects," "intends," "potential," "may," "could," "might," "will," "should," "approximately" or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, included in Intelligent Bio Solutions' public filings filed with the Securities and Exchange Commission. Any forward-looking statements contained in this release speak only as of its date. Intelligent Bio Solutions undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Company Contact:
Intelligent Bio Solutions Inc.
info@ibs.inc
LinkedIn | Twitter
Investor & Media Contact:
Valter Pinto, Managing Director
KCSA Strategic Communications
PH: (212) 896-1254
INBS@kcsa.com
1 Urine results are not included in this chart. Due to the nature of urine sample collection, which depends on an individual's need to urinate, samples cannot be collected at fixed intervals. Therefore, urine specimens were collected on an ad-lib basis as frequently as possible.
A photo accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/6806ebb4-b738-4c65-b0a0-e6a4c1dc9626

Comments