$Eli Lilly(LLY)$ s Alzheimer's disease new drug donanemab (trade name Kisunla) was approved by the FDA. This is an important breakthrough that provides new treatment options for Alzheimer's disease patients. The stock opened lower that day, not because of the news, but because Biden joined Bernie Sanders in "calling" for $Novo Nordisk (NVO)$'s two Netflix diet pills, Ozempic and Wegovy, have had their prices drastically cut, and Eli Lilly has been hit because of its GLP-1 drug Mounjaro.
But it's just shouting, and could be seen as nothing more than a means for Biden, who lost the first debate, to seemingly win favor with American voters. Lilly shares opened lower and higher during the session.
Can this new Alzheimer's drug make a huge difference?
First of all this medication
Scope of application: Early symptomatic Alzheimer's disease patients (not as effective in mid to late stage)
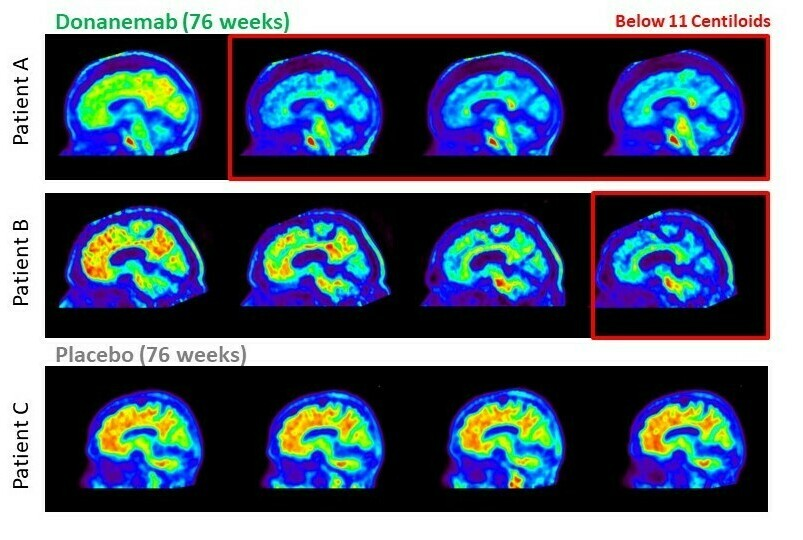
Effects of the medication: The drug slowed the progression of Alzheimer's disease by 35% compared to placebo.
Cost of treatment: monthly intravenous injections cost $12,522 for a 6-month course and $48,696 for an 18-month course. lecanemab costs about $26,500 for annual treatment
Industry Comparison: $Biogen(BIIB)$ and $Eisai Co., Ltd.(ESAIY)$ The company's Leqembi is similar in that both are monoclonal antibodies that slow disease progression by targeting amyloid plaques in the brain.
Side effects: There are some safety risks, such as serious side effects like brain swelling and bleeding.
Comparison between donanemab and lecanemab
Mechanism of action:
Both drugs are monoclonal antibodies that target amyloid plaques in the brain, but donanemab specializes in targeting specific fragments of amyloid for more rapid and complete plaque removal.Mode of Administration:
Donanemab is administered by intravenous injection, whereas lecanemab is administered by subcutaneous injection.Duration of therapy:
Donanemab is designed to be a time-limited therapy, intended to be discontinued after aggressive plaque clearance. lecanemab, on the other hand, requires continuous dosing.Clinical Trial Design:
The pivotal phase III trial of donanemab, TRAILBLAZER-ALZ 2, used the iADRS scale as the primary endpoint.
The phase III trial of lecanemab used the CDR-SB scale as the primary endpoint.Clinical effects.
donanemab: in a population with moderate tau protein levels, slowed decline in iADRS score by 35% (p<0.0001); slowed decline in CDR-SB score by 36% (p<0.0001); slowed decline in ability to perform activities of daily living by 40%; and delayed progression to the next stage of the disease by 39%
lecanemab: 27% slowing of cognitive decline over 18 months.
Safety:
Both drugs carry a risk of ARIA-E (cerebral edema). lecanemab had a 12.5% incidence of ARIA-E in a phase III trial. specific data for donanemab have not been published, but the risk is expected to be similar.Monitoring Requirements:
Donanemab allows physicians to monitor for amyloid re-accumulation after treatment is discontinued. lecanemab requires ongoing dosing and monitoring.Cost of treatment: donanemab is administered intravenously monthly and costs $12,522 for a 6-month course and $48,696 for an 18-month course. lecanemab costs about $26,500 for annual treatment and is already covered by Medicare in the United States.
Overall, donanemab demonstrated stronger cognitive decline mitigation in clinical trials and has potential time-limited therapeutic benefits.
However, due to the limitations of this drug (only applicable in the early stages and delayed by about 30-40%), it is still very difficult to change the problem of Alzheimer's disease on a wide scale, and therefore it can not be a substantial industry-changing drug yet, and only somewhat positively affects the company's revenues and market position.
Comments