Moderna shares rose nearly 2% in premarket trading
Tiger Newspress2022-02-01
Moderna shares rose nearly 2% in premarket trading.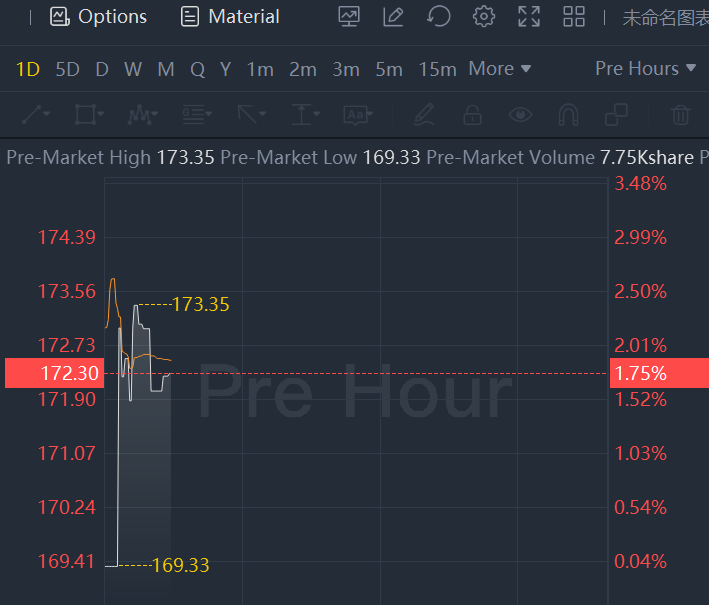 The Food and Drug Administration granted full approval on Monday (Jan 31) to Moderna's coronavirus vaccine, the second-most widely used in the United States and the second to receive full regulatory approval.
The Food and Drug Administration granted full approval on Monday (Jan 31) to Moderna's coronavirus vaccine, the second-most widely used in the United States and the second to receive full regulatory approval.
The vaccine, which can be administered to adults and has been shown to be highly effective at preventing virus infections and severe cases of Covid-19, has been in use for more than a year under an emergency-use authorisation.
That rigorous standard lets federal regulators allow use of the shot in a public health emergency before they complete a longer and more detailed review.
The vaccine received emergency-use authorisation in December 2020.
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.

