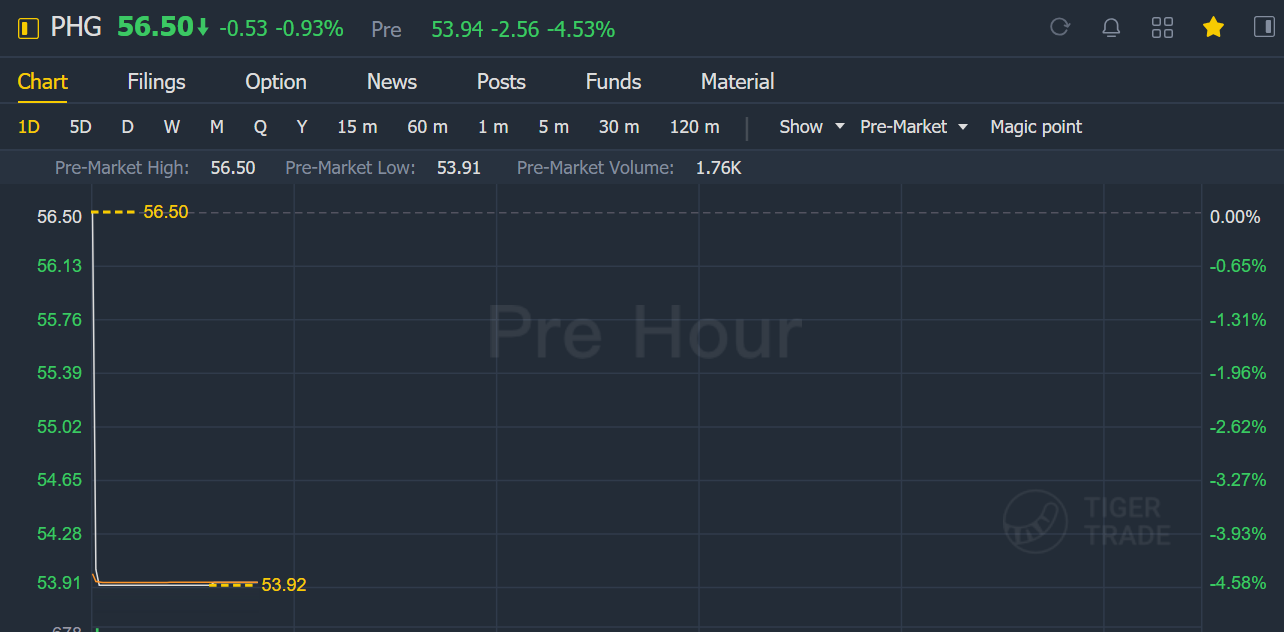
Philips shares fell 4.5% in pre-market trading.
The company said some of its ventilators use sound-abatement foam that may degrade into particles that could be ingested or inhaled and potentially have toxic and carcinogenic effects. Philips doubled its provision for expected costs related to the issue to 500 million euros ($605 million).
The recall is a setback for Philips as it shifts to focus entirely on health-care products. The company reached an agreement earlier this year to sell its domestic-appliances business to private equity firm Hillhouse Capital and first flagged the quality issue with certain sleep and respiratory-care products in April.
Exposure to the polyester-based polyurethane, or PE-PUR, foam that that can enter patients’ airways can cause headache, irritation, inflammation and respiratory issues, according to Philips. The company issued a recall notification only for the U.S., where around 65% of the devices were sold, and a field safety notice for the rest of the world.
