The US FDA has approved Reata Pharmaceuticals' Friedreich's ataxia (FA) treatment omaveloxolone.
Branded as Skyclarys, the drug is approved for those 16 years and older with the inherited neuronal disorder. Liver function tests are required with use.
Reata is up 174% in morning trading.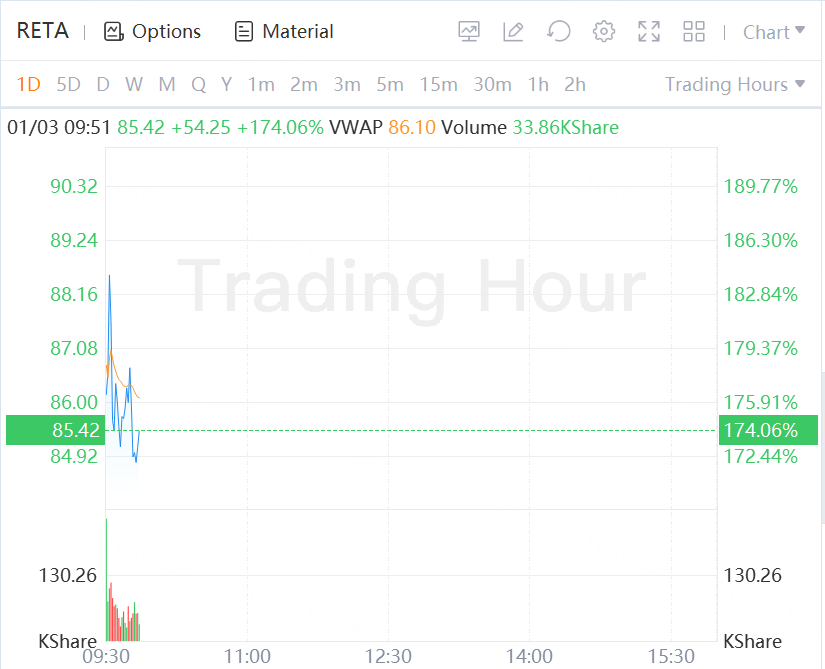
Treating the condition now involves off-label use of SSRI antidepressants, nerve pain drug Lyrica (pregabalin) for pain, and ALS drug riluzole for balance, Sheng-Han Kuo, MD, a movement disorder specialist in neurology at Columbia University Medical Center, told Seeking Alpha.
Kuo, who has done scientific consulting for Reata (RETA), said he would prescribe Skyclarys to all of his FA patients unless they are unable to tolerate it.
Other treatments in development also hold promise for FA, according to Kuo, who is the director of the Initiative for Columbia Ataxia and Tremor.
He mentioned PTC Therapeutics (PTCT) vatiquinone (PTC743), a protein replacement drug to deliver functional frataxin ("FXN") protein, and AAV gene therapy.
Following approval, Reata (RETA) received a rare pediatric disease priority review voucher.
