The Food and Drug Administration has authorized fourth Pfizer and Moderna Covid vaccine dose for everyone age 50 and older, amid uncertainty over whether an even more contagious version omicron will cause another wave of infection in the U.S. as it has in Europe and China.
The FDA also authorized a second Pfizer booster shot for people age 12 and older who have compromised immune systems, and a second Moderna booster for adults ages 18 and older with compromised immune systems. The new boosters are administered at least fourth months after the last shot.
The FDA made the decision without a meeting of its vaccine advisory committee, a rare move the agency has made more frequently over the course of the pandemic to expand uses of Covid vaccines. The drug regulator’s authorization comes just two weeks after Pfizer and Moderna asked the FDA to permit a second booster shot based on data from Israel. The Centers for Disease Control and Prevention is expected to quickly sign off on the decision.
The FDA’s decision effectively bypasses its advisory committee on vaccines, which is scheduled to meet on April 6 to discuss the future of booster shots in the U.S. The vaccine experts are expected to hold a broad discussion about boosters and will not vote on a specific recommendation.
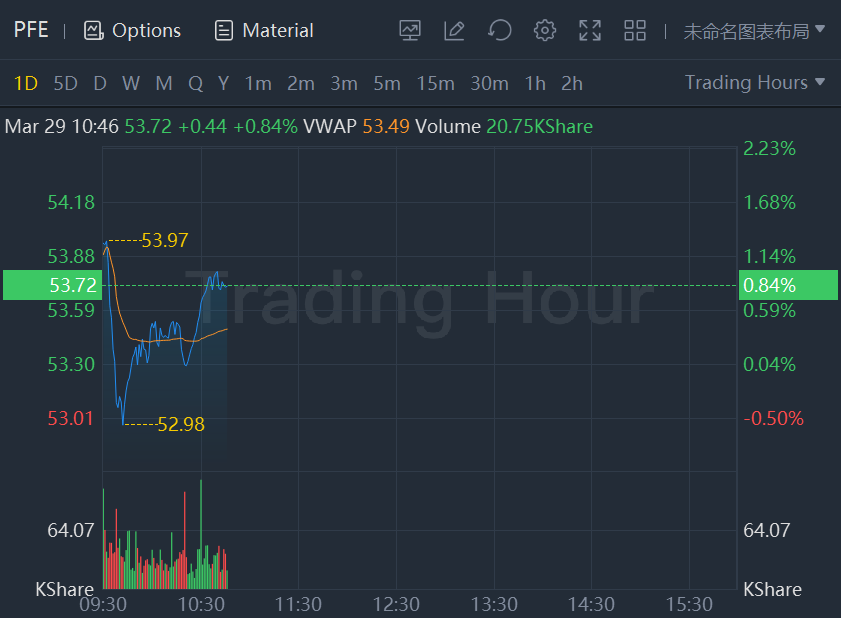
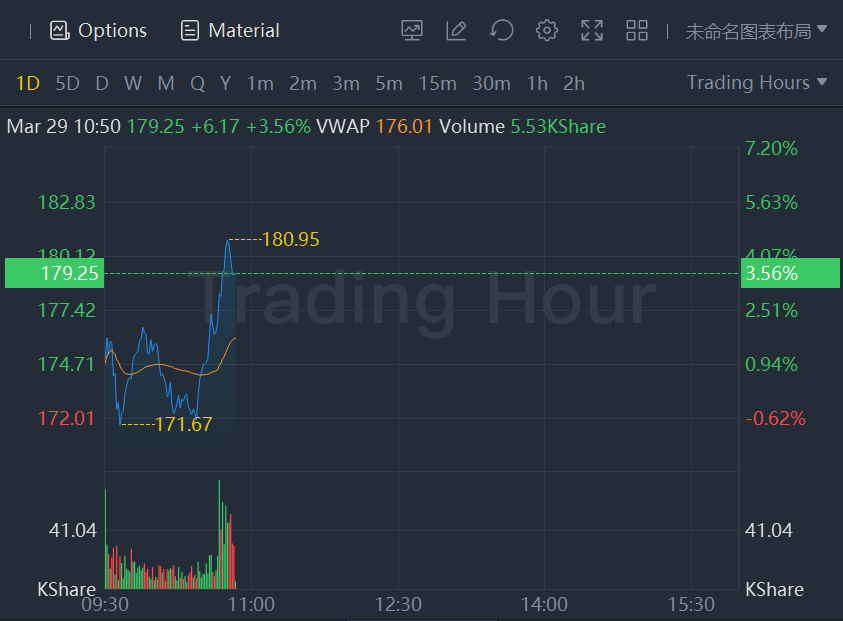
Pfizer shares added nearly 1% while Moderna stock rose more than3% in morning trading.