U.S. FDA Authorizes Novavax's COVID Vaccine As Booster for Adults
Reuters2022-10-19
 Oct 19 (Reuters) - The U.S. Food and Drug Administration on Wednesday authorized Novavax Inc's(NVAX.O)COVID-19 vaccine as a booster for adults.
Oct 19 (Reuters) - The U.S. Food and Drug Administration on Wednesday authorized Novavax Inc's(NVAX.O)COVID-19 vaccine as a booster for adults.
The booster authorization applies to people who are unable to get updated Omicron-tailored boosters, or those who would choose not to receive any other booster dose of a vaccine.
The regulator's decision is in addition to the earlier clearance for the vaccine as a primary two-shot regimen for those 12 years and above.
The company, however, has been struggling with sales of the vaccine and in August had halved its full-year revenue forecast, saying it does not expect further sales of its COVID-19 shot in the United States this year.
Novavax shares jumped 3.44% in morning trading.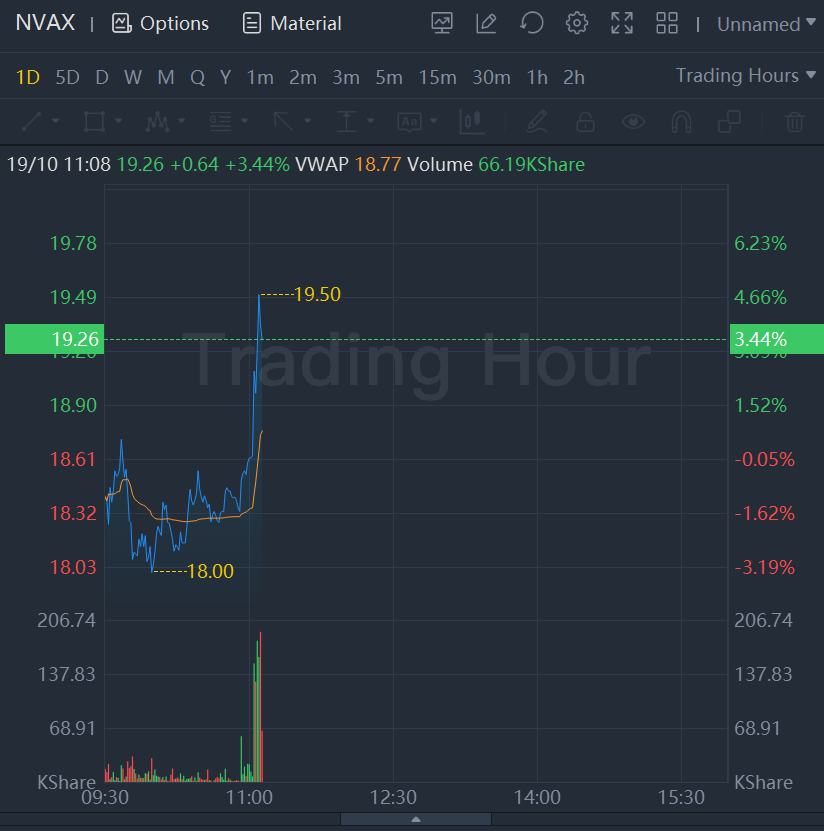
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.


