- Company plunges After Covid Shot Falls Short With 47% Efficacy
- Effectiveness is far below that seen with other mRNA vaccines
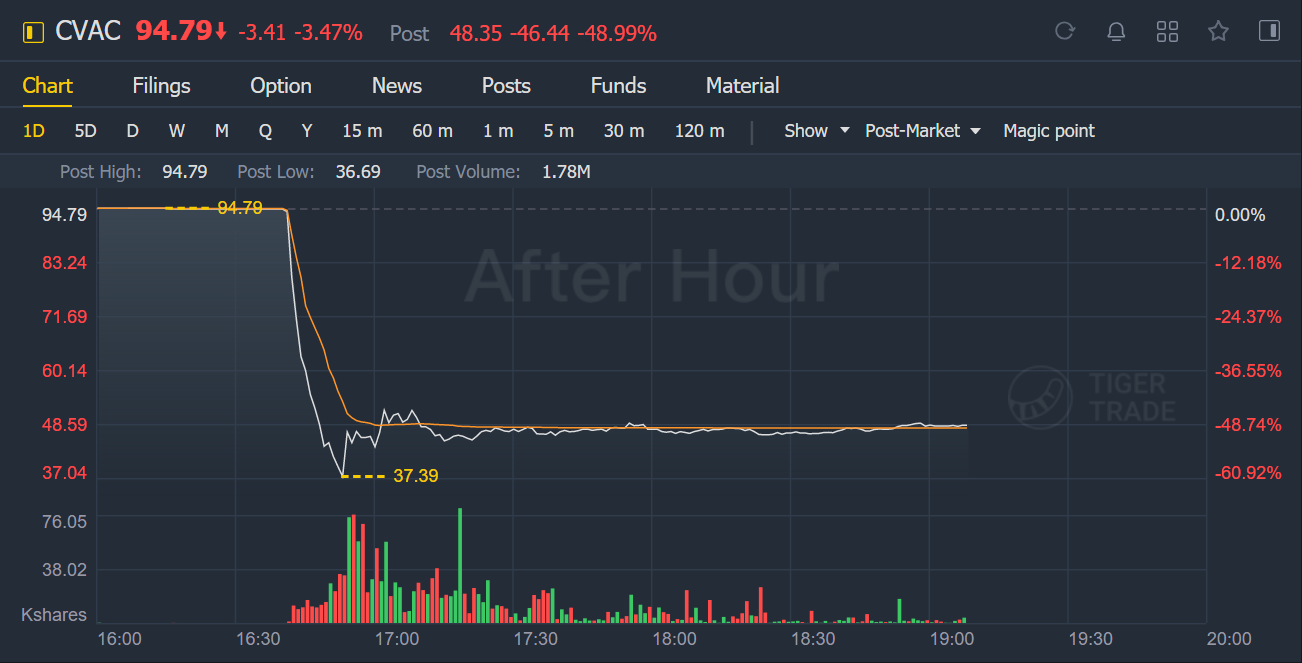
CureVac NV shares plunged after a preliminary analysis of a large study muddied by the spread of virus variants found its Covid-19 vaccine was 47% effective, well short of the high bar set by other messenger RNA shots.
The interim analysis of data from about 40,000 volunteers included 134 Covid cases, the German company said in a statement. CureVac declined to say how many who got the shot got sick or how many received a placebo. But the results suggest the vaccine works less well for older people than in a younger population, Chief Technology Officer Mariola Fotin-Mleczek said in an interview Wednesday.
Though preliminary, the results throw the future of the vaccine into question as wealthy nations around the world move swiftly to inoculate their populations with shots already available. Still, CureVac will finish its trial and plans to gain approval, Chief Executive Officer Franz-Werner Haas said.
“There is a huge need for vaccine, and I think we can still make a contribution,” Haas said.
The company’s shares fell more than 49% in post-market trading after being up 17% this year through Wednesday’s close.
In April, Elon Musk tweeted about CureVac, which Tesla Inc. has partnered with on a vaccine printer,saying it “sounds like they’re a few months away from regulatory approval.” He added that “a tidal wave of vaccine is coming this summer.” He later deleted the tweet.
The shot could be a boost in the developing world, since it doesn’t require the deep freezing needed for other Covid shots. Partly owned by the German government, CureVac has forged partnerships with Bayer AG,GlaxoSmithKline Plc and the U.K. government aimed at speeding production of its shot and future versions that will target mutated strains.
The data comes as the world is at something of a crossroads in the Covid fight, with global infections falling even as outbreaks in places with less access to vaccines serve as a reminder that the pandemic is far from over. The wealthiest countries have taken an outsize share of the more than 2.4 billion shots administered so far.
The proliferation of variants in the CureVac trial is testimony to how the pandemic has changed since the first large vaccine clinical trials were run last year.
Genetic Sequencing
CureVac’s researchers genetically sequenced 124 of the 134 cases they included in their analysis. Just one of the sequenced cases was due to the original SARS-CoV-2 virus first identified in Wuhan, China. The rest came from a range of variants, including 21% of cases attributable to a new variant called lambda that was first identified in Peru.
TheWorld Health Organization added lambda to its watchlist as a variant of interest on Monday.
Some 57% of the Covid cases in the study were due to so-called variants of concern, the four variants that the World Health Organization has deemed the most worrying. Most of these -- about 41% of all the cases -- were due to the alpha variant first observed in the U.K., CureVac said.
That variant is now the most common in the U.S., and other vaccines, including shots from Pfizer Inc. and BioNTech SE as well as Novavax Inc., have shown themselves effective against it. CureVac didn’t release detailed data about how its shot performed against each of the variants.
Final Readout
More details will emerge in the final readout of the study, which will include more than 200 Covid cases and could come within two or three weeks, CureVac said.
Like the shots from Moderna Inc. and the Pfizer-BioNtech alliance, CureVac’s vaccine relies on mRNA, enlisting the body’s own cells as vaccine factories. Unproven until last year, the technology has now catapulted to the forefront of the global fight against Covid.
CureVac’s shot, though, is subtly different than its competitors, because it uses an unmodified form of mRNA.
CureVac has said it plans to supply 300 million vaccine doses this year, enough to immunize 150 million people. Together with partners, it has said it will supply more than 1 billion doses in 2022.