IQVIA: 生物类似药市场
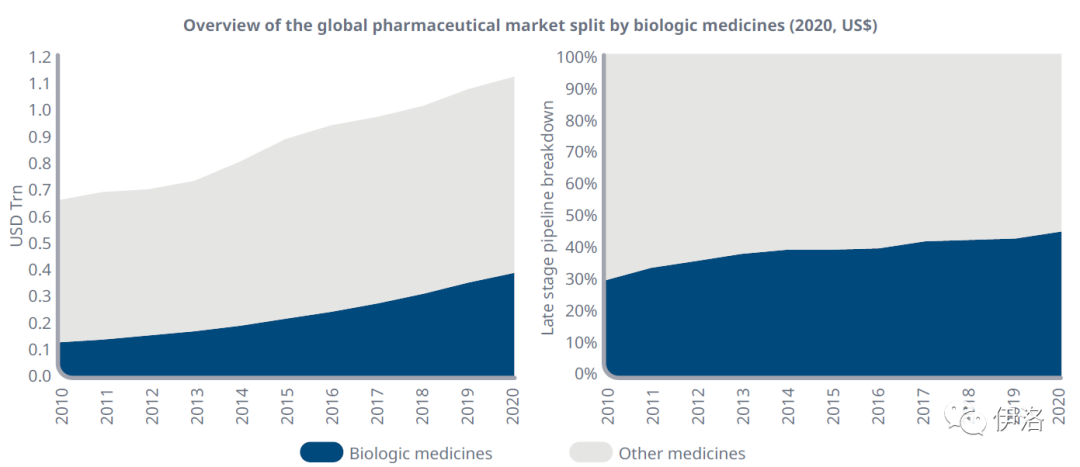
Exhibit 1: Increasing Importance of the Biologic Medicines Market
Source: IQVIA MIDAS® Q4 2020, IQVIA Institute analysis using Pipeline Intelligence
Exhibit Notes: Biologic medicines are defined as innovative biologic molecules and innovative biologics. Other medicines category includes innovative small molecules and generics.
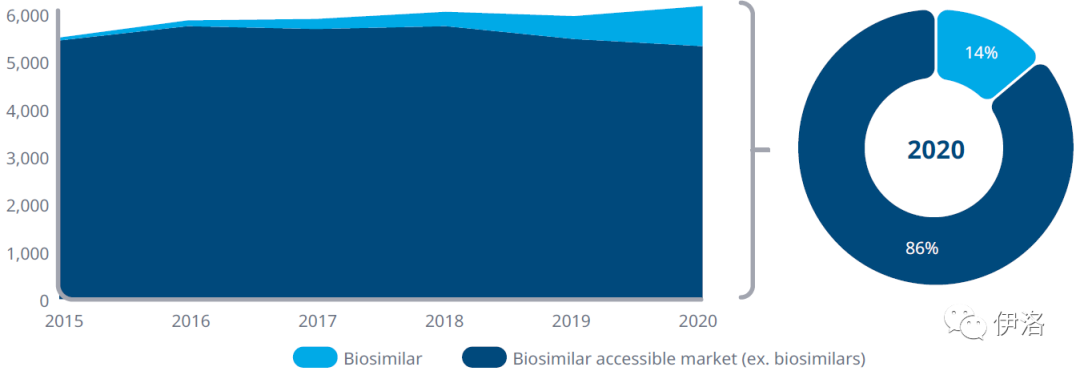
Exhibit 2: Biosimilars as a Portion of the Accessible Biologics Market
European biosimilar share within the accessible market
(treatment days, absolute value, % market share)
Source: IQVIA Impact of Biosimilar Competition Report 2020
Exhibit Notes: Accessible market includes referenced medicines and non-referenced medicines across major therapy areas available for biosimilar competition. Therapy areas include GCSFs, EPO, HGH, fertility, insulins, LLMWHs, anti-TNFS, oncology.
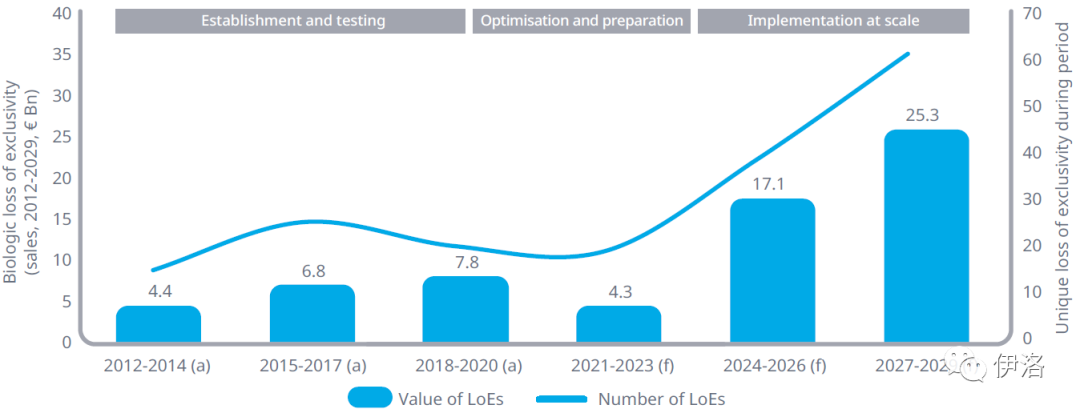
Exhibit 3: Forecast Potential for Biosimilar Competition in Europe
Source: IQVIA MIDAS® Q4 2020, IQVIA ForecastLink data for post-2020 period, Rx biologics in 23 European markets
Exhibit Notes: (a) represents actual sales, (f) = forecast sales. The IP for biologicals can include multiple patents and patent timelines for each individual product and therefore it is difficult to give an exact date for patent expiry of biologics. It should be noted that these results are estimates as determined by IQVIA MIDAS® and ARK Patent Intelligence where available.
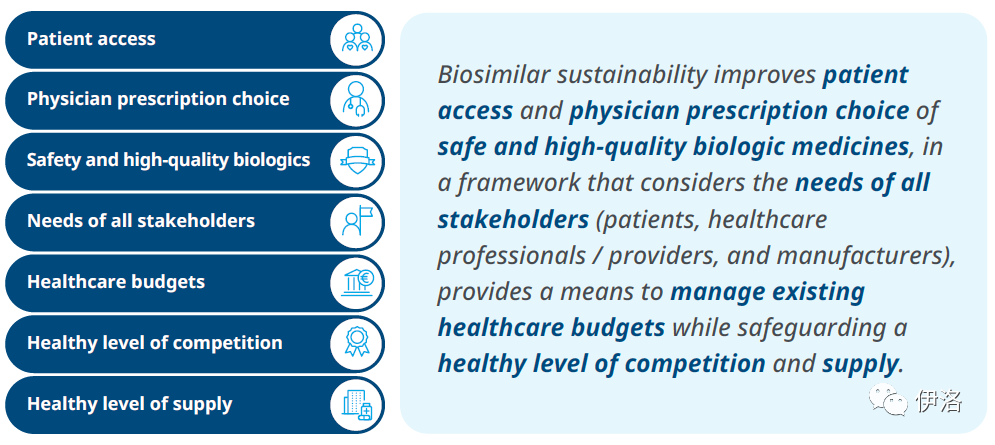
Exhibit 4: Definition of ‘Biosimilar Sustainability’
Source: IQVIA Institute for Human Data Science, Advancing Biosimilar Sustainability in Europe, 2018
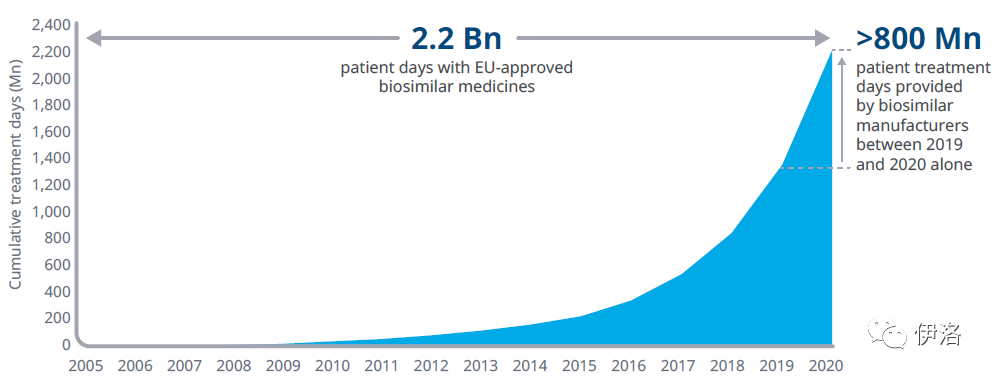
Exhibit 5: Cumulative Patient Treatment Days for EU-Approved Biosimilar Medicines
Source: IQVIA MIDAS® Q4 2020 data
Exhibit Notes: Rituximab and trastuzumab Defined Daily Dose (DDD) calculated via IQVIA Real World Data, Oncology Dynamics physician surveys on average cycles; pre-2009 analysis includes extrapolated treatment days for biosimilars launched between 2005 – 2008; country cohort includes 30 countries within Europe Economic Area; Medicines for Europe estimates September 2017 (700Mn):
https://www.medicinesforeurope.com/news/oncology-the-new-era-for-biosimilar-medicines/
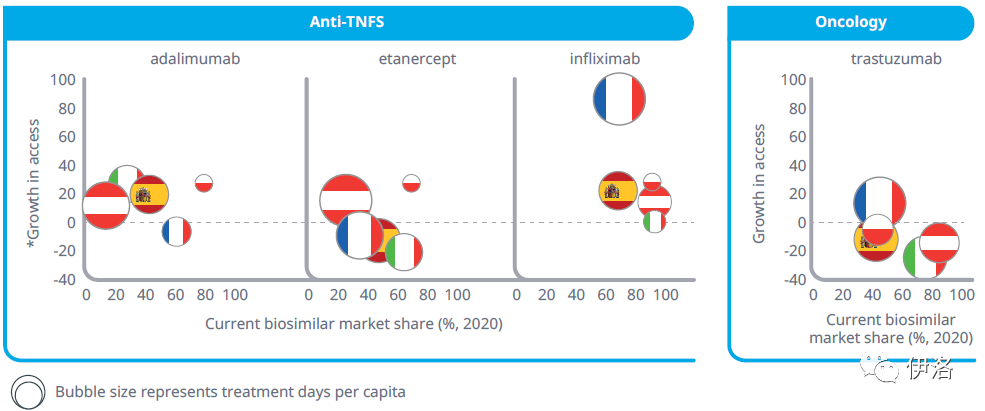
Exhibit 7: The Limited Correlation Between Biosimilar Uptake and Increased Access to Therapy Ordered by Date of Entry
Source: IQVIA MIDAS® Q4 2020 analysis
Exhibit Notes: * = Increase in treatment days per capita versus the year before biosimilar entry
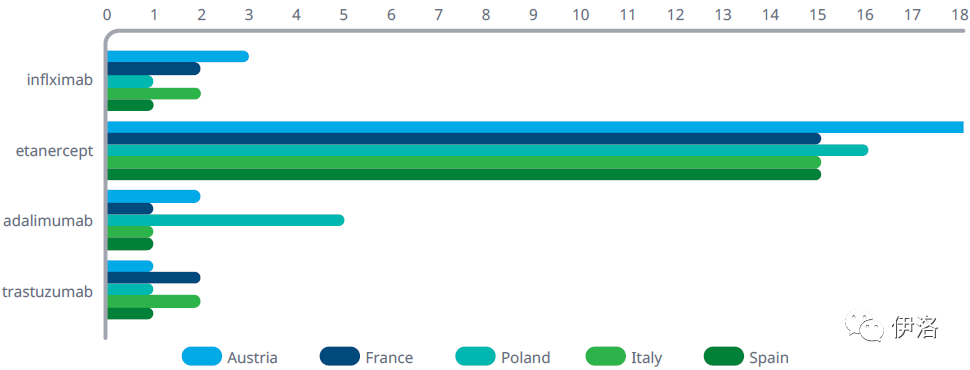
Exhibit 10: Time from EMA Approval to First Sales of Biosimilars (Ordered by Earliest Date of Entry)
Months from biosimilar entry
Source: IQVIA MIDAS® Q4 2020
Exhibit Notes: Biosimilar entry takes into account instances where loss of exclusivity occurs before biosimilars have been authorised for use in Europe, or where sales are present before time to authorisation. The European baseline for biosimilar entry is usually based on the limiting factor of these two components.
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.