Phase Ib/II Data of ALK-1 Combo Study Published in BMC Medicine
Suzhou, October 28, 2023 - Kintor Pharmaceutical Limited (“Kintor Pharma”, HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that the results of the Phase Ib/II clinical trial of ALK-1 antibody GT90001 combined with PD-1 antibody nivolumab in the treatment of advanced hepatocellular carcinoma (HCC) were published online by the well-known journal BMC Medicine (Impact factor: 11.806). The results showed that this combination regimen is well-tolerated and has promising anti-tumor activity in patients with recurrent advanced HCC, with a confirmed overall response rate (ORR) of 30% and a remarkable long-tail effect.
https://doi.org/10.1186/s12916-023-03098-w
HCC is a common pathological type of liver cancer. The early clinical symptoms are not obvious, and 50% of patients are already in the advanced stage when diagnosed. Systemic therapy is recommended for advanced HCC. In recent years, the treatment of advanced HCC has changed from targeted therapies to immunotherapy combination therapies, and atezolizumab combined with bevacizumab has become the new first-line standard of care with an increase in overall survival and progression-free survival rate. Some studies have shown that the therapeutic benefit, in terms of both survival and objective response, may be further improved by combining immune checkpoint inhibitors (ICIs) such as PD-1/ PD-L1 with anti-angiogenic targeted therapy[1,2,3,4]. GT90001 is a fully humanized monoclonal antibody that inhibits ALK-1/TGF-β signal transduction and tumor angiogenesis. It is a potentially first-in-class antibody that we obtained an exclusive global license from Pfizer, Inc.
This Phase Ib/II clinical study is led by Professor Chiun Hsu from the National Taiwan University School of Medicine Hospital. It is a single-arm, open-label, multi-center clinical trial (NCT03893695) designed to evaluate the safety and antitumor activity of GT90001 combined with nivolumab in patients with advanced HCC. This study consisted of a dose de-escalation stage to determine the recommended Phase II dosage (RP2D) of GT90001 in combination with nivolumab and a dose expansion stage to evaluate the safety and efficacy of GT90001 in combination with nivolumab.
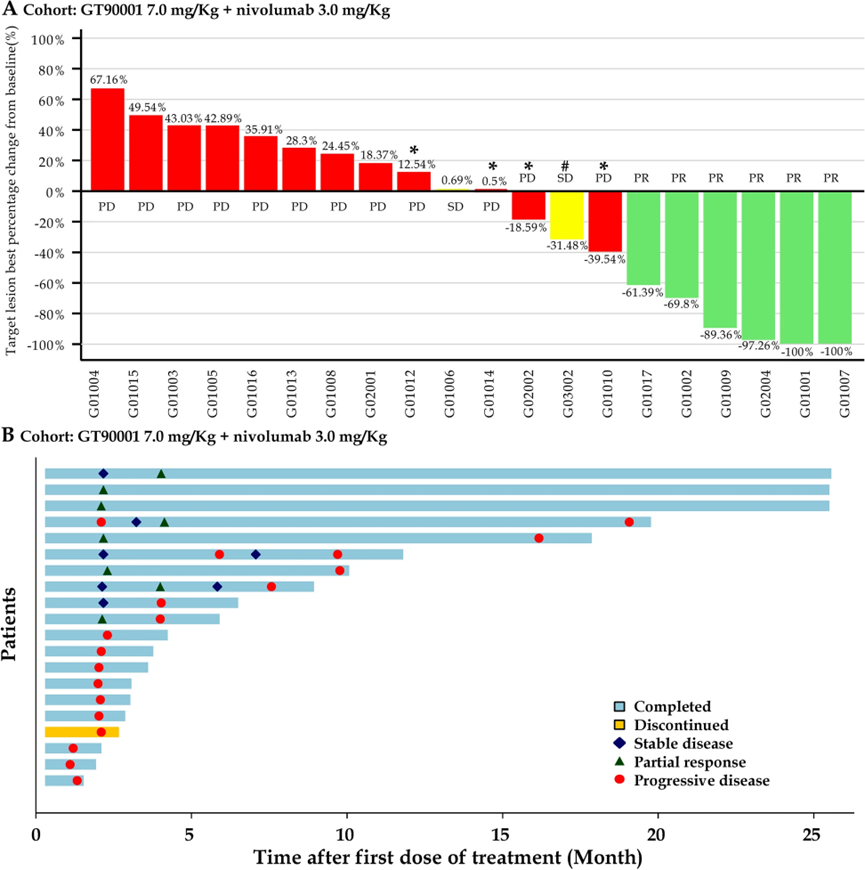
Between 9 July 2019, and 8 August 2022, 20 patients were treated (6 patients in Phase Ib; and 14 patients in Phase II) and evaluated for analysis. The results showed that,
The common grade 3/4 adverse events (AEs) were platelet count decreased (15%). No deaths due to AEs were reported. In Phase Ib, no dose-limiting toxicities were observed, and GT90001 7.0 mg/kg was confirmed as the RP2D.
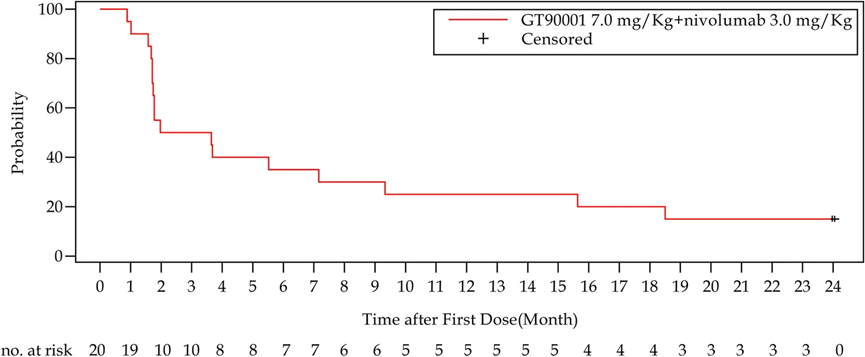
The confirmed objective response rate (confirmed ORR) and disease control rate (DCR) were 30% (95% CI, 14.6%-51.9%) and 40% (95% CI, 21.9%-61.3%), respectively. The median duration of response was not calculated (95% CI, 7.39 months to not calculated). Median progression-free survival (mPFS) was 2.81 months (95% CI, 1.71–9.33), with 6-month and 12-month PFS rates of 35% and 25%, respectively.
Furthermore, the combination of GT90001 and nivolumab showed a remarkable long-tail effect, since 8 subjects were still alive as of 19 April 2023. 6 of them had received no further systematic anti-tumor therapy after trial completion. And 3 patients were still on progression-free status without any other anti-tumor treatment.
This Phase Ib/II study confirmed that the combination of GT90001 (7.0 mg/kg, every 2 weeks) and nivolumab had a manageable safety profile in patients with advanced HCC. Additionally, the results demonstrated promising anti-tumor activity and showed durable remissions and objective responses in this population. This finding suggested that this combination might be a potential treatment option for advanced HCC.
Reference
1.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. J Hepatol. 2022;76(4):862–73.
2.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Lancet Oncol. 2021;22(7):977–90.
3.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. J Clin Oncol. 2020;38(26):2960–70.
4.Yoo C, Ryoo B-Y, Kim H-D, Ryu M-H, Kang B, Chon HJ, et al. J Clin Oncol. 2022;40(4_suppl):415.
About Kintor Pharmaceutical Limited
Kintor Pharmaceutical Limited is developing and commercializing a robust pipeline of innovative small molecule and biological therapeutics for oncology and androgen-receptor-related disease areas with unmet medical needs, including alopecia, acne, COVID-19, prostate cancer, liver and breast cancers. For more information, visit www.kintor.com.cn.
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.