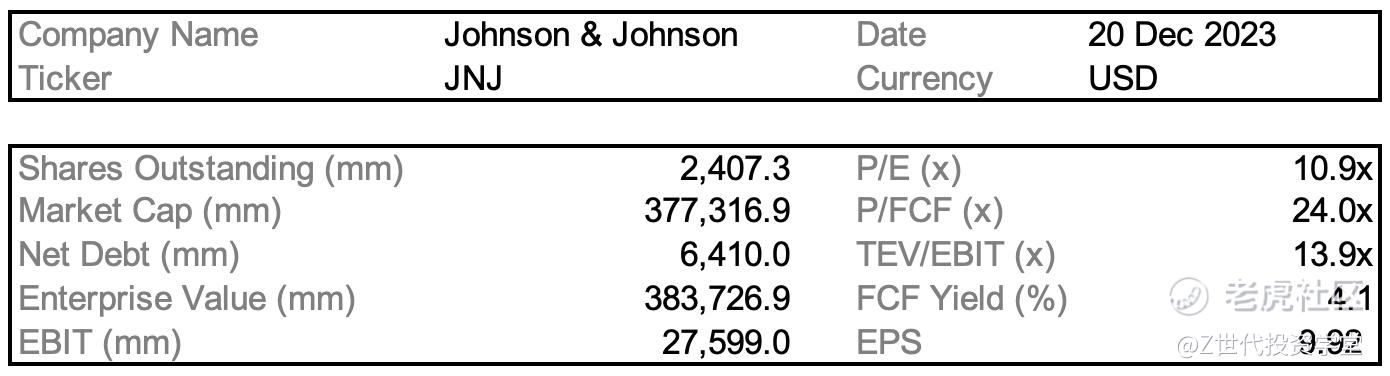
Initial Report(part1): Johnson & Johnson (JNJ), 212.61% 5-yr Potential Upside (EIP, Xinying CHAN)
Executive Summary
Founded in 1886 and headquartered in the U.S., Johnson & Johnson (JNJ) is a multinational corporation operating in healthcare, pharmaceuticals, and consumer goods. The company is known for its diverse product range, including prescription drugs, medical devices, and consumer healthcare products. JNJ has recently separated its consumer health segment into a new entity called Kenvue, leaving two main segments: Pharmaceuticals and MedTech, while maintaining a global reputation for innovation, research, and corporate social responsibility with a focus on sustainability initiatives.
Company Overview
2.1 Business segments
JNJ was originally organised into three business segments: Consumer Health, Pharmaceutical and MedTech. The Consumer Health segment includes a broad range of products used in the Baby Care, Oral Care, Skin Health/Beauty, Over-the-Counter pharmaceutical, Women’s Health and Wound Care markets. These products are sold online and to retail outlets and distributors throughout the world.
The Pharmaceutical segment is focused on areas such as Immunology, Infectious diseases, Neuroscience, Oncology, and Metabolic diseases. Products in this segment are distributed directly to retailers, wholesalers, distributors, hospitals and healthcare professionals for prescription use.
The MedTech segment includes a broad portfolio of products used in the Orthopaedic, Surgery, Interventional Solutions and Vision fields. These products are distributed to wholesalers, hospitals and retailers, and used principally in the professional fields by physicians, nurses, hospitals, eye care professionals and clinics.
2.2 Revenue drivers
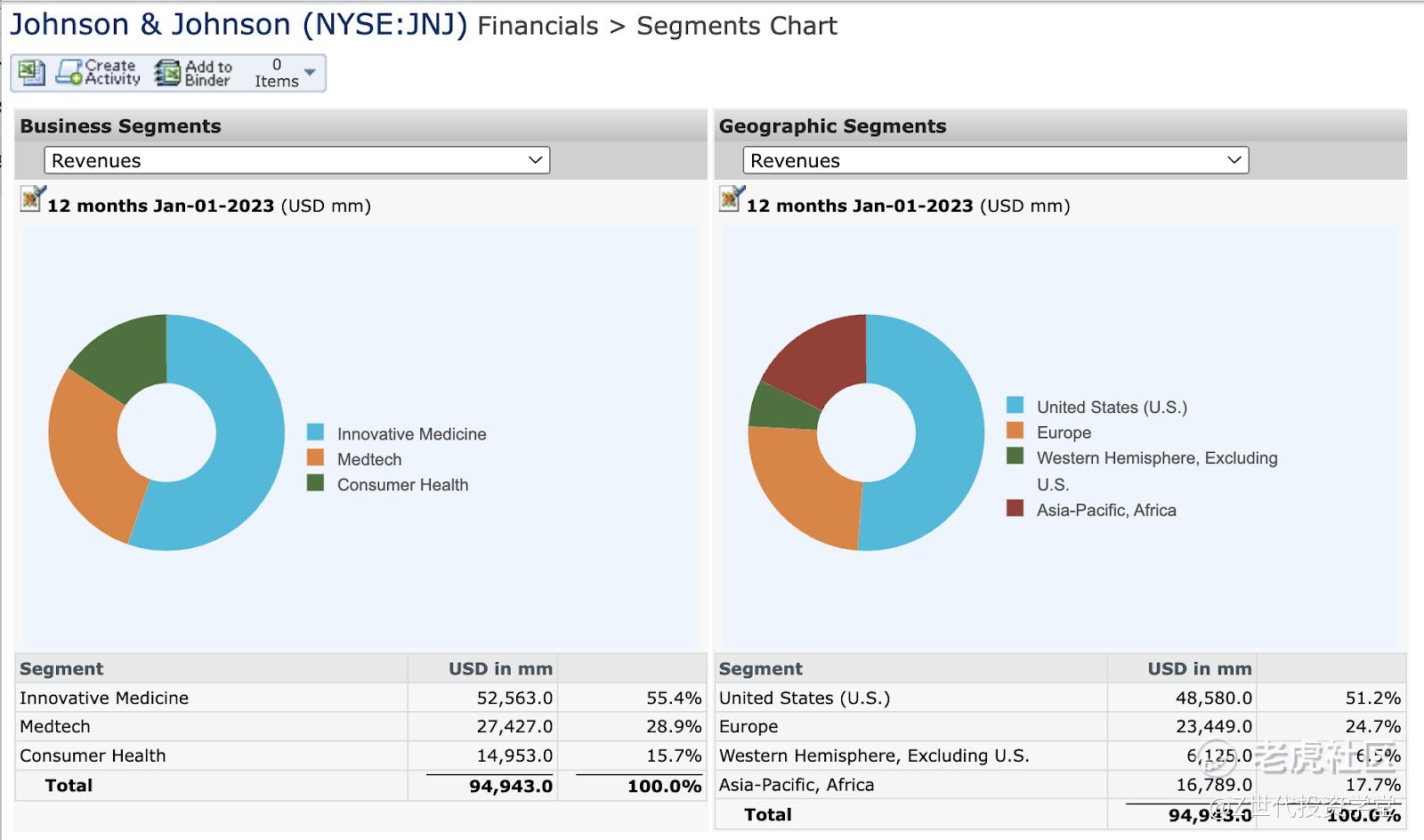
The following chart shows JNJ 2022 revenue by business segments and geographic segments, where the company had about half of its revenue from the United States. As for the revenue breakdown by business segments, slightly more than half was from the Pharmaceutical (Innovative Medicine) business segment, while the Consumer Health segment accounted for only about 15% of total sales.
2.3 Cost drivers
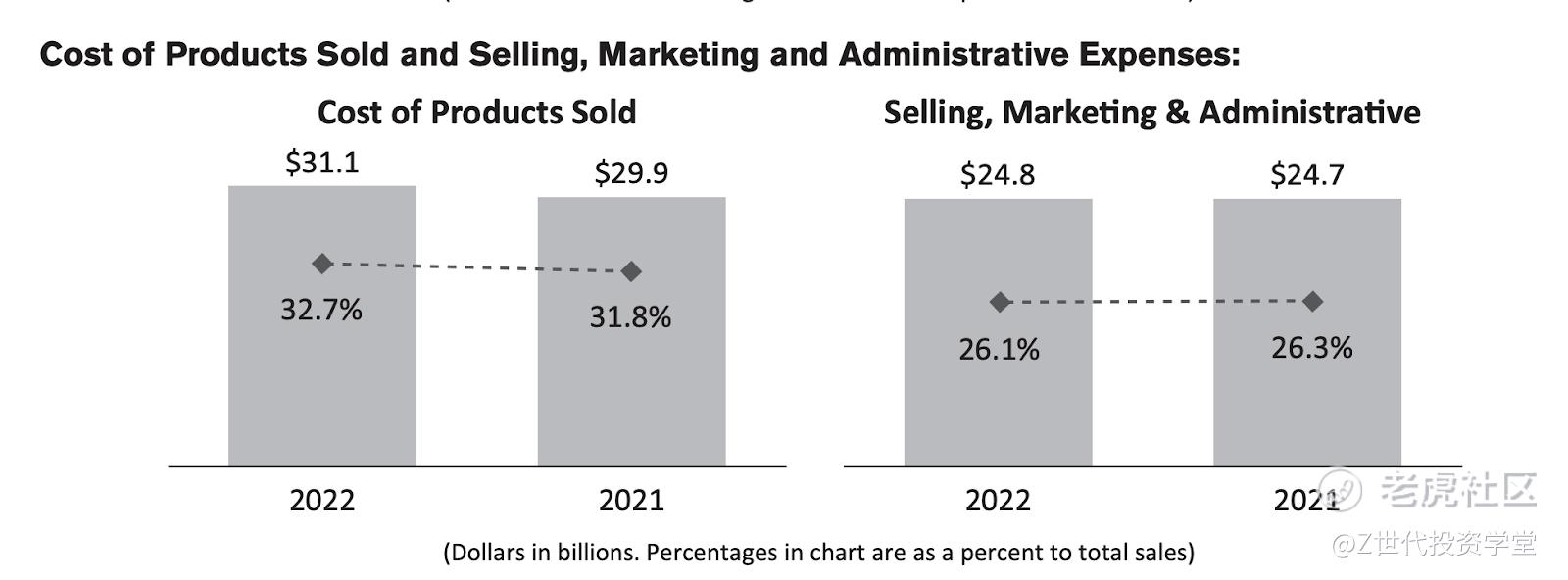
The cost of products sold increased as a percent to sales from 2021 to 2022 was driven by a one-time COVID-19 vaccine manufacturing exit related costs and also currency impacts in the pharmaceutical segment since the company had a global presence.
The exit related costs from manufacturing COVID-19 vaccine, include remaining commitments and obligations, including external manufacturing network exit costs and required clinical trial expenses. On the other hand, due to the strength of the US dollar in the past 2 years, various aspects of the pharma industry, including revenues, international expansion, research and development costs were impacted negatively. For example, in early 2021, the US dollar strengthened against a range of currencies, which impacted the revenues of pharma companies that generate a significant portion of its revenue in other currencies.
Selling, Marketing and Administrative Expenses decreased as a percent to sales due to a reduction of brand marketing expenses in the Pharmaceutical and Consumer Health businesses.
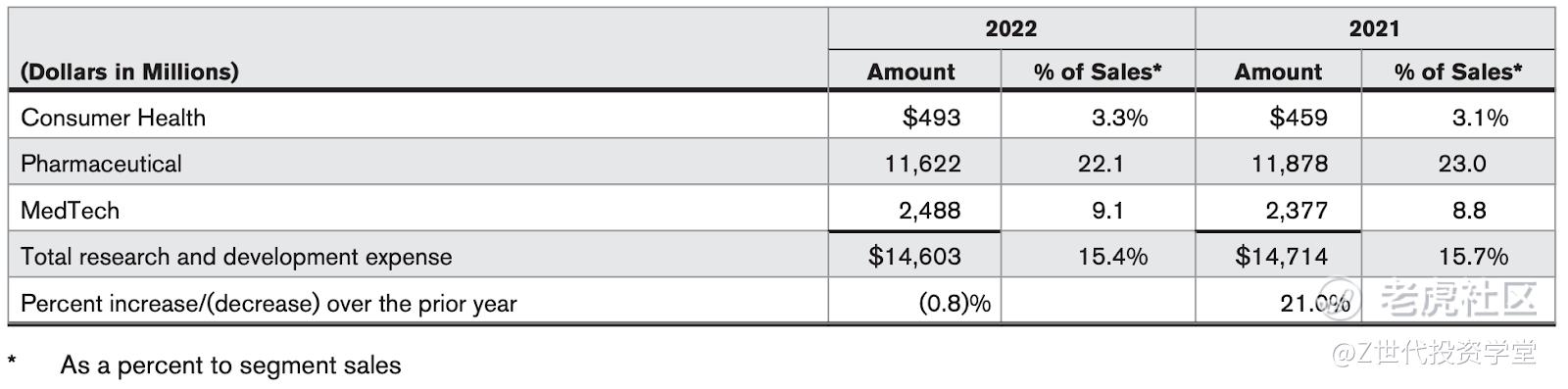
Research and development expense by segment of business was as follows:
The company also dedicates a significant portion of its business in its research and development activities. These expenditures relate to the processes of discovering, testing and developing new products, upfront payments and developmental milestones, improving existing products, as well as ensuring product efficacy and regulatory compliance prior to launch. From 2021 to 2022, research and development decreased as a percent to sales primarily driven by lower milestone payments in the pharmaceutical segment.
2.4 ESG considerations
JNJ's materiality table highlights consumer health and patient safety, product quality, and access as the three most material topics for the company.
Based on MSCI's industry-specific material topics, product safety and quality, along with governance, emerge as the two most material topics for the pharmaceutical industry. Additionally, according to MSCI ESG ratings, JNJ is also in the high average 79 companies in the pharmaceutical industry.
2.4.1 Environmental
JNJ significantly expanded procurement of renewable energy globally, propelling JNJ towards 100% renewable electricity goal at the global level by 2025. New initiatives in 2022 included Power Purchase Agreements (PPAs) and Utility Green Tariffs, as well as expansion of on-site solar generation in several countries. JNJ maintains more than 50 on-site renewable energy systems in 20 countries and has executed more than 15 contracts for off-site renewable electricity procurement.
In addition, JNJ Vision removed the plastic pouches on the outside of delivery boxes for all ACUVUE contact lenses in Europe, saving tons of plastic across Europe every year, while in the UK, JNJ Vision’s ACUVUE Contact Lens Recycle Scheme launched in 2019, has collected over 8.7 million lenses and recently expanded to increase capacity of lenses, blister packs and foils to be recycled each year.
2.4.2 Social
The company has improved its performance in terms of providing access to healthcare. It has also demonstrated leadership in access to medicines, moving up to second place in the 2022 Access to Medicine Index (ATMI) which provides insights into how the world's leading pharmaceutical companies perform on ensuring people living in low- and middle-income countries have access to the medicines, vaccines and diagnostics it needs.
JNJ also made a $800 million investment in the Healthy Lives Mission through a range of initiatives promoting skin cancer awareness, supporting smoking cessation, advocating for menstrual dignity, expanding product transparency and improving packaging sustainability across a wide range of Consumer Health brands in line with commitments as a signatory to the Ellen MacArthur Foundation’s New Plastics Economy Global Commitment.
Furthermore, the company commenced a yearlong global refresher program of its Six Safety Habits framework for all employees in our R&D spaces and supply chain facilities to reacquaint them with the workplace habits that ensure safety continues to be an essential part of its culture.
2.4.3 Governance
JNJ’s Board of Directors constitutes six standing committees: the Audit Committee, the Compensation & Benefits Committee, the Nominating & Corporate Governance Committee, the Finance Committee, the Regulatory Compliance & Sustainability Committee, and the Science & Technology Committee. The committee members are made up of 13 board of director members with diverse backgrounds ranging from healthcare to education.
The Science & Technology Committee is led by independent directors, focusing on shaping and monitoring policies for environment, health, safety, and sustainability. Simultaneously, the Regulatory Compliance & Sustainability Committee, also comprising independent directors, oversees the effective implementation of the company's healthcare compliance, ethics, and quality programs. Both committees play vital roles in upholding the company's commitment to environmental responsibility and ethical healthcare practices.
With regards to medical safety, JNJ made advancements in multiple initiatives to improve medical safety, including innovative real-world data (RWD) methods to generate reliable real-world evidence about patient health outcomes across numerous initiatives spanning pharmaceutical products, vaccines, medical devices and Consumer Health products. One of the initiatives in 2022 was improving access to critical medical devices using RWD by generating extensive evidence of comparative safety of critical devices.
Competitor Analysis
Pfizer Inc. is a global biopharmaceutical company involved in the comprehensive process of discovering, developing, manufacturing, and distributing medicines and vaccines across various therapeutic areas. Its diverse product portfolio includes treatments for cardiovascular, metabolic, migraine, women's health, infectious diseases, and COVID-19 prevention and treatment. Pfizer is also engaged in biosimilars, addressing chronic immune and inflammatory diseases, as well as conditions like amyloidosis, hemophilia, endocrine diseases, and sickle cell disease. The company, founded in 1849, is headquartered in New York, New York.
Eli Lilly and Company, founded in 1876 and based in Indianapolis, Indiana, is a global pharmaceutical company with a focus on discovering, developing, and marketing human pharmaceuticals. Its product range covers diabetes medications like Basaglar, Humalog, and Humulin, as well as offerings for cancer treatment such as Alimta, Cyramza, and Retevmo. Eli Lilly has also contributed to COVID-19 treatments, including Bamlanivimab and etesevimab.
Novartis AG is a Swiss-based healthcare company established in 1996 and headquartered in Basel. It engages in the research, development, manufacturing, and marketing of prescription medicines globally. The company's therapeutic focus includes cardiovascular, renal and metabolic conditions, immunology, neuroscience, oncology, ophthalmology, and hematology. Notably, Novartis has a licence and collaboration agreement with Alnylam Pharmaceuticals for the development, manufacturing, and commercialization of inclisiran, a therapy designed to reduce LDL cholesterol. The company emphasises providing healthcare solutions and collaborates with physicians.
Modify on 2024-04-01 22:49
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.