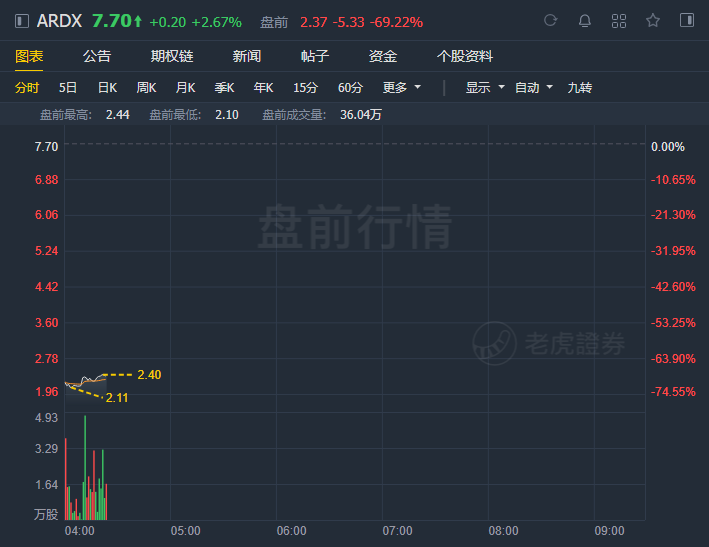
Shares in Ardelyx Inc. (ARDX) plummeted nearly 70% in premarket trading Tuesday, following the biopharmaceutical company's announcement that the Food and Drug Administration appears unlikely to approve a drug for dialysis patients.
Ardelyx revealed that it received a letter from the FDA stating that deficiencies in the information provided had been found that would preclude discussion of approvals Ardelyx has sought.
When Ardelyx sought a meeting with the FDA to discuss the deficiencies, the request was denied, though Ardelyx stated that "the FDA noted that a key issue is the size of the treatment effect and its clinical relevance."
"This is an extremely disheartening and disappointing communication from the FDA, particularly following the weeks of label discussions that occurred in early April, the fact that our NDA submission included three pivotal trials across 1,000 patients, all which met their primary and key secondary endpoints, as well as the additional data analyses we submitted in late April in response to the FDA's requests," Ardelyx Chief Executive Mike Raab said in a statement.
Ardelyx shares, which closed at $7.70 in the regular session for a market capitalization of more than $700 million, saw shares dive to less than $2.50 in the after-hours trading period.